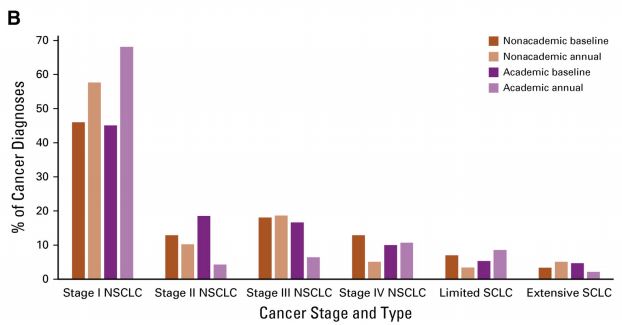
GO2 for Lung Cancer, Dana-Farber Cancer Institute, and Addario Lung Cancer Medical Institute Announce Expansion of Landmark Patient Research on Underlying Causes of Inherited Risk of Lung Cancer
Clinical Trial Aims to Unlock the Origins of Lung Cancer WASHINGTON and BOSTON, April 17, 2024 – In a promising step forward, GO2 for Lung Cancer (GO2), Dana-Farber Cancer Institute (Dana-Farber), and the Addario Lung Cancer Medical Institute (ALCMI), GO2’s medical research consortium, announced a major expansion of landmark [...]
Fern Halper, Ph.D., Appointed to GO2 for Lung Cancer Board of Directors
Brings background in data, AI, and analytics to help guide research collaboration and improved patient outcomes WASHINGTON, April 15, 2024 — GO2 for Lung Cancer (GO2) announced that the organization elected Fern Halper, Ph.D. to its board of directors. Halper is vice president and senior director of TDWI Research for advanced [...]
From Patient to Advocate: David’s Story
David lives in Arlington, VA, with his wife, Lisa, son, Ethan (6), and daughter, Liv (3). He is a passionate supporter of the Baltimore Ravens, Washington Capitals, Baltimore Orioles, and Penn Men’s Lacrosse, of which he was captain in 2007. He and his wife are in the process of [...]
GO2’s 5K Walk/Run Series Welcomes the White Ribbon Project
We are excited to welcome the White Ribbon Project as a 2024 5K walk/run partner. Representatives from the White Ribbon Project will be on-site at our 5Ks to share their important mission and distribute white ribbons to community members who would like to help raise awareness about the disease. We [...]
Ask the Experts: Women and Lung Cancer
Question: My daughter was just diagnosed with lung cancer at the age of 35. Her doctor told us that many young women are now getting lung cancer. Why is this? (Answered by Dr. Narjust Florez from the Dana-Farber Cancer Institute during her appearance at the February 2024 Lung Cancer [...]
Ask the Experts: Radiation Therapy
Question: My mom was recently diagnosed with lung cancer, and her healthcare team recommended radiation as part of her treatment. We don’t know much about radiation yet. What are the different types of radiation therapy, and how are they given? Is it difficult to deliver radiation to a tumor [...]
2024 Lung Cancer Support Group Facilitator Awardee, Christine Conti
Congratulations to our 2024 Lung Cancer Support Group Facilitator award recipient, Christine Conti, from Huntington Health in Pasadena, California. This award, given annually since 2009, recognizes the uncommon dedication of support group facilitators. Christine Conti, RN, ONN-CG, is a thoracic nurse navigator who has been Huntington Health’s lung cancer support [...]
“My Job is More Than a Job”
Author: Meg Fay, BSN, RN, Nurse Manager, Excellence in Healthcare Delivery, GO2 for Lung Cancer My name is Meg, and I had the pleasure of experiencing the NYC Marathon 2024 as part of the GO2 Endurance Team. Coach Seth guided our team, offering multiple training programs that we could tailor [...]
2024 Lung Cancer Voices Summit Recap
GO2 for Lung Cancer’s Voices Summit is the only annual meeting that brings the lung cancer community together for education, training, connection, and advocacy action with our federal government, the largest funder of cancer research. This month, nearly 200 advocates from 31 states gathered in Washington, D.C., for the [...]
How One Survivor Became an Advocate: Perspectives from a Voices Summit First-Timer
Earlier this month, Oakland-based photographer Genevieve joined nearly 200 other lung cancer advocates in Washington, D.C., to share her story with Congressional members and urge support for lung cancer research. We talked to her about her experience as one of 109 first-time participants at the GO2 for Lung Cancer [...]
Living Room Show Notes: Women and Lung Cancer
Women living with or at risk for lung cancer often have different, and sometimes worse, experiences than men with the same disease. This is due in part to biological differences between men and women and in part to the historical exclusion of women in clinical trials. This has resulted [...]
Ask the Experts: What Does it Mean to Develop Resistance to Treatment?
Question: I was recently diagnosed with lung cancer and am on my first line of treatment. I keep hearing that at some point I may develop a resistance to that treatment. What exactly does that mean and how long might I have before resistance develops? (Answered by Dr. Jyoti [...]
Mary’s Lung Cancer Story: It Doesn’t Stop You From Living
For people diagnosed with lung cancer, receiving a second opinion can be an important step in confirming treatment decisions or opting for a new approach to treatment. In Mary Burlie’s case, getting a second opinion changed everything. Although her first oncologist did not believe that Mary’s lung cancer had [...]
FDA Approves Rybrevant (amivantamab-vmjw) for Non-small Cell Lung Cancer with EGFR-Exon 20 Mutation
On March 1, 2024, the U.S. Food and Drug Administration (FDA) approved the drug Rybrevant (amivantamab-vmjw) to be given in combination with carboplatin and pemetrexed in the first lining setting for people with metastatic non-small cell lung cancer (NSCLC) with an EGFR exon 20 mutation. They also announced traditional approval [...]
GO2 for Lung Cancer Holds Lung Cancer Voices Summit on Capitol Hill to Advocate for Increased Research Funding for the Leading Cause of Cancer Death
More than 230,000 Americans will die of lung cancer this year WASHINGTON, March 4, 2024 - On Tuesday, March 5 GO2 for Lung Cancer (GO2) will host more than 200 supporters on Capitol Hill to educate members of Congress on the urgent needs of the lung cancer community. GO2’s Lung Cancer [...]
Meet HealthUnlocked’s “Denzie:” Celebrating 13 Years of Survival
If you have spent any time in GO2’s online support community, HealthUnlocked, you have likely come across posts, advice or support from a member who calls herself “Denzie.” Denzie volunteers as a dedicated moderator, welcomes new users, answers questions and provides encouragement and comradery to [...]
Ask the Experts: What’s New in SCLC Research?
Question: I have small cell lung cancer (SCLC). What’s new in research related to SCLC? What should I know about clinical trials? (Answered by Dr. Jacob Sands from Dana-Farber Cancer Institute during his appearance on the Lung Cancer Living Room. It has been edited slightly for this use.) Answer: [...]
Finding Hope in an ALK+ Diagnosis
Craig S. lives in Washington, D.C., with his wife, Lydia, and daughter, Lola, who is a freshman at Tulane University. He has worked in software sales and sales leadership since getting his MBA from George Washington University. He enjoys rooting for the Washington Nationals, cooking, [...]
Do I Need Another Test? The Importance of Repeat Biomarker Testing in NSCLC
Author: Brittney Nichols, MPH, BSN-RN, Senior Specialist, Science & Research, GO2 for Lung Cancer Biomarker testing is an important part of the cancer diagnosis and care continuum. For almost all people diagnosed with non-small cell lung cancer (NSCLC) biomarker testing is considered a routine part of guideline-concordant care (the [...]
Unleashing Precision: AI’s Promising Role in Revolutionizing Lung Cancer Care
Author: Matthew Reiss, MSE, PhD, Manager, Precision Medicine & Navigation, GO2 for Lung Cancer You’ve likely started to see artificial intelligence (AI) being used more and more in your everyday life. While the benefits and challenges of using AI in fields such as healthcare must be carefully considered, we [...]
Antibody-Drug Conjugates (ADCs): A Promising Treatment for Lung Cancer
Author: Renee Botello, MSc, Navigator, Treatment and Trials, GO2 for Lung Cancer With decades of research and advances in precision medicine, scientists continue to broaden the treatment standards and options for patients with non-small cell lung cancer (NSCLC). By developing new ways, strategies, and innovative research techniques, this rapid [...]
Nicole Phipps Is Spearheading GO2’s Efforts to Confront Lung Cancer Every Day for Everyone
Nicole Phipps took a job working in lung cancer advocacy almost thirteen years ago to honor her grandfather, a lung cancer survivor. Now she’s applying that passion—and her considerable skills—to ensure that vital lung cancer education, support, connection, and community are accessible to all. Meet [...]
Fern Halper, PhD: Data, AI, and Its Promise for People With Lung Cancer
Fern Halper, PhD, vice president and senior director of TDWI Research and GO2 board member Fern Halper, PhD has a lifelong passion for data. She has spent more than a decade collecting, managing, and analyzing large amounts of data for a Fortune 500 company and another two [...]
FDA Approves Tepmetko (tepotinib) for People with Metastatic Non-Small Cell Lung Cancer with MET-Exon 14 Mutation
On Feb. 15, 2024, the U.S. Food and Drug Administration (FDA) granted traditional approval of Tepmetko (tepotinib) for people living with metastatic non-small cell lung cancer (NSCLC) with a MET-Exon 14 mutation. Tepmetko (tepotinib) initially received accelerated approval in 2021 based on initial results from the VISION study, which [...]
Lung Cancer is the Leading Cause of Cancer Death in Women—GO2 for Lung Cancer President & CEO Provides Testimony as Part of the “Legislative Proposals to Support Patients and Caregivers” Hearing
WASHINGTON, Feb. 16, 2023—GO2 for Lung Cancer President and CEO Laurie Ambrose submitted testimony to the House Committee on Energy and Commerce, Subcommittee on Health. Ambrose’s testimony was in support of the Women and Lung Cancer Research & Preventive Services Act of 2023, introduced in the House by Congressman [...]
GO2 for Lung Cancer Appoints Courtney Granville, Ph.D., M.S.P.H. as Chief Scientific Officer
Granville brings experience overseeing scientific strategy and research WASHINGTON, Feb. 12, 2024 — GO2 for Lung Cancer (GO2) announced that it has hired Courtney Granville as chief scientific officer. In this role, she oversees GO2’s community-engaged research, clinical research, Lung Cancer Registry, and LungMATCH programs. She develops and manages the implementation [...]
Faith, Family, and a Groundbreaking Trial
Aundrea H. is a married mother of three who lives in Washington state, where she homeschools her children (ages 17, 15, and 12). She enjoys spending time with her family and friends as well as being involved in church activities. My diagnosis In December 2023, I was recovering [...]
Living Room Show Notes: What To Do if Resistance Develops
Many people being treated for lung cancer will eventually develop resistance to their current therapies, causing them to stop working. When this happens, and a person’s cancer starts to progress on their current treatment, their healthcare team will take a tissue and/or liquid biopsy to access their new treatment [...]
Ask the Experts: Do I Need a Bronchoscopy?
Question: My doctors discovered something abnormal on a scan of my lungs that they suspect may be lung cancer. I’ve been told that I need a bronchoscopy to biopsy the growth to confirm this diagnosis and determine the stage of my cancer. Do I still need to do this [...]
GO2 Joins National Leaders Calling on the White House to Expand Women’s Health Research and Funding
GO2 for Lung Cancer joined a diverse group of women’s health champions by signing two letters sent to First Lady Jill Biden, who leads the White House Initiative on Women’s Health Research. The letters offer insights and recommendations on opportunities to fundamentally change approaches to increased health research and [...]
Ask the Experts: Support Groups
Question: There is not a lung cancer support group near me, and I would like to start one. What do I do? Answer: You have come to the right place! For decades, GO2 has monitored lung cancer-specific support groups across the country, so we know where to refer people [...]
A New Perspective: Ellen’s Story
Ellen S. is a three-year survivor of stage 4 (IV) non-small cell lung cancer. She retired two years ago after a 22-year career as an occupational therapist in New York. She loves to travel and hike and has visited Ecuador, Costa Rica, Cambodia, Morocco, Iceland, and many states in [...]
Top Five Living Room Episodes of 2023
In 2023, you—the lung cancer community—watched our Lung Cancer Living Room episodes more than 43,000 times! We know that every episode resonates with different people who are living with or at risk for lung cancer depending on their current experiences or interests, but five rose to the top as [...]
Meet the Honorees: 18th Annual Simply the Best Gala
For the past 18 years, we have held our Simply the Best Gala with survivors, loved ones, providers, researchers, and industry partners, to celebrate and honor outstanding members of the lung cancer community. “We are grateful to the many dedicated individuals that bring tangible hope to people at risk [...]
Adedayo Adeniyi: Making an Impact One Policy at a Time
Adedayo Adeniyi was drawn to public health advocacy because he knows how one piece of legislation can change the lives of many. Adedayo, who has a degree in public health and a master’s in public policy, is GO2 for Lung Cancer’s government affairs manager, where he is responsible for [...]
Ask the Experts: Insurance Coverage for Lung Cancer Treatment
Question: My insurance does not cover the cost of my lung cancer treatment. What are my options? Answer: When insurance does not cover the cost of lung cancer drug treatments, there are a few options to explore for financial assistance. Get to know your insurance: We know dealing with [...]
Living Room Show Notes: Our Year of HOPE
For our last Lung Cancer Living Room of 2023, host and GO2 for Lung Cancer Chief Patient Officer, Danielle Hicks, led a conversation looking back at GO2’s growth and impact over the past 12 months. Titled “Our Year of HOPE,” this episode detailed some of the important programs and [...]
2023 Year in Review
Author: Laurie Fenton Ambrose, GO2 for Lung Cancer CEO, President, and Co-Founder It seems like only yesterday that we raised a toast to 2023, and yet another year is rapidly winding down as we find ourselves preparing for a wonderful holiday season, surrounded by family and friends celebrating special [...]
The Power of Advocacy
Sven de Jong wants elected officials to see the faces of lung cancer. Sven de Jong knows that personalizing lung cancer matters. After his wife Elizabeth was diagnosed with stage 4 (IV) ALK+ non-small cell lung cancer in September 2016, the couple was determined [...]
Find Hope, Help, and a Home
Amita Jain supports GO2 for Lung Cancer to help others, too. Shortly after Amita Jain was diagnosed with stage 4 (IV) lung cancer in January 2019, her then-teenage daughter went looking for information and found GO2 for Lung Cancer. She and her father started attending the monthly Lung Cancer [...]
Ask the Experts: Nutrition During Treatment
Answered by Michele Szafranksi, MS, RD, CSO, LDN, Clinical Nutrition Manager, Section of Oncology Nutrition, Department of Supportive Oncology, Levine Cancer Institute Question: I was diagnosed with lung cancer a few weeks ago and am about to start treatment. What role can nutrition play in helping me to feel [...]
Advancing the Frontiers of the Field: Bonnie Addario
Since her own diagnosis 18 years ago, our co-founder, Bonnie J. Addario, has made it her mission to ensure that every person living with lung cancer has a voice representing their needs. Bonnie began her activism by founding the Addario Lung Cancer Foundation, now GO2 for Lung Cancer, to [...]
Living Room Show Notes: Lung Cancer Awareness Month (LCAM) Edition
During November for Lung Cancer Awareness Month (LCAM), GO2 for Lung Cancer and the LCAM Coalition hosted a special Lung Cancer Living Room to discuss stigma. The LCAM Coalition raises awareness about lung cancer and the need for more research, screening, and treatment options to improve survival. Lung [...]
Treatments and Study Updates: Highlights from ESMO 2023
Contributing Authors: GO2 for Lung Cancer Associate Director, Clinical Research, Andrew Ciupek, PhD and Manager, Precision Medicine & Navigation, Matthew Reiss, MSE, PhD Each year, researchers, physicians, and industry partners gather at the European Society for Medical Oncology (ESMO) Congress to share the latest updates on new treatments [...]
Sydney Barned Wants You to Join Her at the Lung Cancer Voices Summit
Sydney Barned, MD, has marked the date on her calendar: March 3–5, 2024. That’s when lung cancer advocates nationwide will unite in D.C. for GO2’s next Lung Cancer Voices Summit. She’s passionate about advocacy and wants more people, especially younger women, to join her in [...]
Ask the Experts: All About Biomarkers
Question: I was just diagnosed with lung cancer. My doctor told me that I should have biomarker testing done to determine the best treatment for me. What are biomarkers, and why will testing for them affect my treatment options? Answer: “Biomarkers” are mutations or changes inside cancer cells that [...]
Living Room Show Notes: Medical and Radiation Oncologists
Medical and radiation oncologists are important members of your lung cancer care team who work together with you to determine your treatment plan and deliver your care. Drs. Jonathan Riess and Megan Daly from UC Davis’s Comprehensive Cancer Center joined GO2’s October Lung Cancer Living Room to discuss the [...]
GO2 for Lung Cancer Celebrates the Lung Cancer Community at the 18th Annual Simply the Best Gala
SAN CARLOS, Calif. and WASHINGTON (November 13, 2023) – GO2 for Lung Cancer (GO2) honored champions of lung cancer at its 18th Annual Simply the Best Gala held in San Francisco on November 11. This special evening was an opportunity to recognize industry partners, researchers, doctors, nurses, survivors, funders, and individual supporters [...]
FDA Approves Augtyro (repotrectinib) for People with Locally Advanced or Metastatic ROS1-Positive Non-Small Cell Lung Cancer
On Nov. 16, 2023, the U.S. Food and Drug Administration (FDA) approved Augtyro (repotrectinib) for people living with locally advanced or metastatic non-small cell lung cancer (NSCLC) with a ROS1 gene mutation. The approval was based on results from the TRIDENT-1 study, which evaluated the use of Augtyro (repotrectinib) [...]
Heather Law Is Looking to Crack the Code on Lung Cancer
Seeking insights from the causes of lung cancer to support for family caregivers Heather Law, MA, is seeking answers. She wants to understand why some people get lung cancer and others don’t. She’s anxious to learn how treatments change over time and what improves a person’s quality of life. [...]
Cliff Norton Brings Hope, Determination to Lung Cancer Advocacy
When Cliff Norton walks into a room, he brings hope through the door with him. Diagnosed with stage 4 (IV) lung cancer in July 2010, he was declared NED (no evidence of disease) in December 2012. More than a decade and many PET scans later, he’s still cancer-free. His [...]
Rick Sherlock Brings a Life of Service to the Lung Cancer Community
Rick Sherlock has spent his life in service to others. The retired army major general has flown Chinooks, Blackhawks, and other aircraft. He has served his country at home and abroad, including tours of duty in South Korea, Iraq, and Germany, as well as several tours of duty at [...]
Groundbreaking Research on Inherited Gene Mutation in Lung Cancer Published in Journal of Clinical Oncology
GO2 for Lung Cancer announced that researchers have described a new familial syndrome in people with lung cancer caused by an inherited gene mutation. The research study was published this month in the Journal of Clinical Oncology. The report chronicles the methods and findings of the INHERIT study, more than [...]
GO2 for Lung Cancer Launches PSA Featuring Actor and Director Tony Goldwyn for National Lung Cancer Awareness Month in November
Goldwyn has a personal connection to lung cancer WASHINGTON, November 1, 2023— GO2 for Lung Cancer, is launching a public service announcement campaign starting the first of November in conjunction with National Lung Cancer Awareness Month. The PSA features acclaimed actor, producer and director Tony Goldwyn, recently seen in the blockbuster [...]
Ask the Experts: Surgery for Lung Cancer
Question: I’ve just been diagnosed with lung cancer, and my doctor said I may need to have surgery. What can I expect? Answer: Your healthcare team will explain the best type of surgery for you, but the goal will be to remove all of the tumor(s) without removing too [...]
Do You Speak Spanish?
Haga clic aquí para leer esta página en español. GO2 for Lung Cancer is excited to expand its Spanish-language offerings, and we need your help! If you have been diagnosed with lung cancer, or are a caregiver for someone with the disease, and are frustrated by the lack of [...]
Presidential Proclamation Designates November 2023 as National Lung Cancer Awareness Month
As we come together on the eve of Lung Cancer Awareness Month, we are excited to share that President and First Lady Biden have joined us again to bring much-needed attention to this disease by issuing a Presidential Proclamation designating November 2023 as National Lung Cancer Awareness Month. As in [...]
Treatments, Trials, and Technology in Lung Cancer
Author: GO2 for Lung Cancer Specialist, Science & Research, Brittney Nichols MPH, BSN-RN Each year, scientists and doctors from across the globe gather at IASLC’s World Conference on Lung Cancer (WCLC) to discuss the latest and greatest in lung cancer treatment and research. New treatment options on the horizon [...]
New Clinical Trial Designs in the Era of Precision Medicine
Author: GO2 for Lung Cancer Associate Director, Clinical Research, Andrew Ciupek, PhD With an increasing number of targeted therapies becoming available, making treatment decisions based on the results of biomarker testing has become an essential part of cancer care – especially in lung cancer. At the same time, researchers [...]
Addressing the Unmet Needs of Family Caregivers of People with Lung Cancer
Author: Lung Cancer Registry Associate Director, Heather Law, MA Family caregivers often experience emotional distress and neglect their own well-being while caring for a loved one. Preliminary findings from GO2’s Lung Cancer Registry Caregiver Survey were presented at the 2023 World Conference on Lung Cancer (WCLC) in Singapore in September. Understanding the unmet need Advances in diagnostic [...]
Perspectives from Providers, Patient Advocates, and People Living with Lung Cancer
Author: GO2 for Lung Cancer's Specialist, Science & Research, Brittney Nichols MPH, BSN-RN The IASLC annual World Conference on Lung Cancer (WCLC) unites lung cancer professionals from across the world to connect and share insights. This goes beyond the science and medicine of treating and diagnosing lung cancer—it expands [...]
Lung Cancer After Tagrisso Resistance: What Next?
Author: GO2 for Lung Cancer Treatment and Trials Navigator, Renee Botello MSc Tagrisso (osimertinib) has been the treatment of choice and effective standard of care option for the treatment of EGFR+ non-small cell lung cancer (NSCLC) since its FDA approval in 2018 as a first-line treatment for EGFR+ NSCLC. [...]
ASCO Issues New Recommendation Cosela (Trilaciclib) in Update to its Treatment Guidelines for Small Cell Lung Cancer
On October 11, 2023, the American Society of Clinical Oncology (ASCO) recommended an update to its treatment guidelines for small cell lung cancer (SCLC). Cosela (trilaciclib) has been recommended as a myeloid supportive agent for individuals with extensive-stage SCLC who are receiving treatment with chemotherapy or chemoimmunotherapy. Myelosuppression or bone [...]
FDA Approves New Treatment Regimen for Individuals Receiving Keytruda (pembrolizumab) for Resectable Non-Small Cell Lung Cancer
On October 16, 2023, the U.S. Food and Drug Administration (FDA) approved a new treatment plan for people receiving Keytruda (pembrolizumab) for resectable non-small cell lung cancer (NSCLC). This approval is based on results from the KEYNOTE-671 (NCT03425643) clinical trial that assessed a new treatment plan for persons with [...]
FDA Approves Combination Braftovi (encorafenib) and Mektovi (binimetinib) for Metastatic BRAF-Mutated Non-Small Cell Lung Cancer
On October 11, 2023, the U.S. Food and Drug Administration (FDA) approved a combination of Braftovi (encorafenib) and Mektovi (binimetinib) for people with metastatic non-small cell lung cancer (NSCLC) with a BRAF V600E mutation. BRAF is a less common genetic mutation, affecting 1-2% of individuals diagnosed with NSCLC, and [...]
Living Room Show Notes: What’s New in Pulmonary Care
“If you can’t breathe, nothing else matters.” This powerful statement was shared by Dr. D. Kyle Hogarth from the University of Chicago during September’s Lung Cancer Living Room to emphasize the importance of having a pulmonologist on your lung cancer care team. A pulmonologist is a medical professional who [...]
Ask the Experts: I’m Worried About Bothering My Doctor. What Should I Do?
Question: I have many questions about my lung cancer diagnosis and treatment options, but I’m worried about bothering my doctor. What should I do? Answer:* If you have an oncologist who makes you feel like you shouldn’t be contacting them, then you have the wrong oncologist. What will keep me [...]
A Laugh a Day
Author: Dr. Jennifer K. Renshaw, OTD, OTR/L, OTA/L There are many words for laughter: chuckling, giggling, snickering, hooting, hollering, and howling. But is laughter really the best medicine? Whether you are a person living with lung cancer or a caregiver, laughter may be a resource to consider. According to [...]
A Passion for People: How Jennifer Hughes Confronts Lung Cancer
Jennifer Hughes is getting ready for a party next month. And not just any party, but GO2 for Lung Cancer’s “Simply the Best” gala. Hughes, senior director of national events, oversees every detail of GO2’s gala, which raises awareness and resources to confront lung cancer. It’s [...]
Finding Strength After a Small Cell Lung Cancer Diagnosis: Bob’s Story
After spending over 20 years in the hospitality field, Bob Iles changed careers and joined the stock brokerage industry before retiring almost six years ago. He and his husband live in Palm Springs, CA, where he enjoys swimming in his lap pool, hiking, biking, volunteering for environmental and LGBTQ+ [...]
Ask the Experts: Neo-Adjuvant vs. Adjuvant Therapy
Question: What is the difference between neo-adjuvant therapy and adjuvant therapy? Answer: “Neo-adjuvant” and “adjuvant” are terms used in the context of cancer treatment to refer to therapies that are given before or after the primary treatment, such as surgery or radiation therapy. Neo-adjuvant therapy refers to treatment given before [...]
Support for Small Cell Lung Cancer
GO2 for Lung Cancer’s Small Cell Lung Cancer (SCLC) program offers education, support, and connection to those affected by SCLC. People living with SCLC often have different needs than those with non-small cell lung cancer (NSCLC). Our SCLC program aims to better meet those needs by expanding our SCLC-specific resources, [...]
Top Treatment Takeaways from the World Conference on Lung Cancer
Every year, lung cancer professionals from across the globe gather to connect and learn at the World Conference for Lung Cancer (WCLC). Members of the GO2 team just returned from WCLC 2023 hosted in Singapore, where they presented GO2’s work as well as learning about the latest treatment updates. [...]
A New Lease on Life: Mike’s Story
By Mike Knecht Mike Knecht retired from his career as an electrical contractor and business owner in 2019 and spent the following year traveling through the United States and Africa. In early 2023, he and his wife, Lois, sold their home in Maryland to live full-time in their vacation/retirement home in West Virginia. Mike enjoys a wide variety of activities from hobby farming on his 150-acre mountain-top property, to visiting his daughters and grandchildren on both [...]
Scanxiety: Tips from a Survivor and Expert
By Alison Mayer Sachs, MSW, LSW, OSW-C, FAOSW, Director, Community Outreach & Cancer Support, Services, Eisenhower Lucy Curci Cancer Center, Eisenhower Medical Center and lung cancer survivor “Scanxiety” is a term that people with cancer use to describe the anxiousness and fear they experience before, during, and after scans to [...]
Living Room Show Notes: New Age of Small Cell Lung Cancer
Small cell lung cancer (SCLC), which represents about 15% of all lung cancer diagnoses, is an often-aggressive form of lung cancer that has historically been difficult to treat. However, the science driving new treatments for SCLC is moving faster than ever before, with more drugs in development right now than [...]
Ask the Experts: At Stage 1A, Do I Need Chemo?
Question: A small 2 cm nodule was recently found in my lung and I had an upper lobectomy. The surgeon said the margins were clear and there was no cancer found in the lymph nodes. They said the stage is IA (1A) and scheduled me for a scan in [...]
Processing Trauma After a Lung Cancer Diagnosis
By Susan Smedley Susan Smedley is a 25-year lung cancer survivor and former social worker who worked in the national lung cancer community for 15 years, most recently at GO2 for Lung Cancer. As a yoga instructor trained in oncology and trauma-informed yoga, she retired from nonprofit work to [...]
GO2’s Joelle Fathi Wants to Transform Healthcare
Joelle Fathi is on a mission to make a meaningful contribution and transform healthcare to make it more equitable. It’s an ambitious goal that GO2 for Lung Cancer’s chief healthcare delivery officer believes is essential to increase lung cancer survivorship in the long term. We caught up with Fathi [...]
Survivor Spotlight: Presley A.
My name is Presley and I was diagnosed with stage 4 (IV) non-small cell lung cancer on Sept. 19, 2022. It felt like the rug had been pulled out from under me. I was only 34 years old, a wife, and a mother of four children all under the [...]
Donor Spotlight: Jana B.
Jana Brownell is the Chairman of the Kirlin Company and has been a GO2 for Lung Cancer supporter since 2001. My mom was diagnosed with non-small cell lung cancer (NSCLC) in 2001. At the time of my mom’s startling and unimaginable diagnosis, we were focused on my dad, who [...]
GO2 Receives a $250,000 Award
GO2 for Lung Cancer to Receive a $250,000 Engagement Award to Build Capacity for Patient Engagement in Research Within a Stigmatized Lung Cancer Community San Carlos, CA and Washington, DC – Today GO2 for Lung Cancer (GO2) announced its Chief Healthcare Delivery Officer, Dr. Joelle Fathi, DNP RN, ARNP, CTTS, [...]
Stronger Together: GO2 for Lung Cancer and KRAS Kickers
GO2’s Phone Buddy Program, first launched in the 1990s, is a peer-to-peer matching program that pairs people who have been diagnosed with lung cancer to share stories, hope, and wisdom with one another. As a GO2 Phone Buddy herself, Terri Conneran, founder of KRAS Kickers, knows the value of bringing [...]
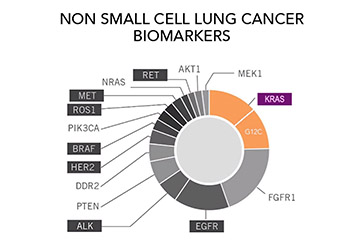
Ask the Experts: Clinical Trials for KRAS+ Lung Cancer
Question: I have KRAS+ lung cancer. Are there any clinical trials available for me? Answer: Yes! No matter what kind of lung cancer you are diagnosed with, there is likely a trial appropriate for you. GO2 for Lung Cancer has our own KRAS study — launched with our medical research consortium, [...]
Meet GO2’s New National Ambassador Council Members
Bob Nicklas, nine (9) year lung cancer survivor and GO2 NAC member Two new advocates joined GO2 for Lung Cancer’s National Ambassador Council (NAC) and participated in their first meeting last month. Bob Nicklas joins NAC after receiving GO2’s 2022 Voices Summit Leadership & Advocacy Award, serving [...]
Living Room Show Notes: Best of ASCO 2023
The American Society of Clinical Oncology (ASCO) hosts an annual meeting every spring where oncologists and other physicians, researchers, industry members, and advocacy organizations like GO2 come together to share and learn the latest and most exciting news on emerging science in the cancer field. While ASCO covers all [...]
Screening Saves Lives: Barney’s Story
By Barney Brinkmann Barney Brinkmann retired four years ago after a 40-year career with AT&T as an engineer in St. Louis and Dallas. His sense of wanderlust has taken the St. Louis native to India (five times!); scuba diving off Tioman Island, the Hawaiian Islands, and the South Pacific; [...]
Ask the Experts: Lung Cancer Screening
Question: My children want me to talk to my doctor about being screened for lung cancer. How do I know if I’m eligible and what should I expect? Answer: Annual lung cancer screening is recommended for people whose age and smoking history put them at higher risk for lung [...]
GO2 for Lung Cancer Honors David Carbone, MD, PhD with the 2023 Bonnie J. Addario Lectureship Award
SAN CARLOS, Calif. and WASHINGTON, D.C. (July 28, 2023) – GO2 for Lung Cancer (GO2) announced today that it presented David Carbone, MD, PhD with the 2023 Bonnie J. Addario Lectureship Award for his work developing treatments for lung cancer. GO2 honored Dr. Carbone with the award at the 24th International Lung Cancer Conference [...]
ASCO 2023: Surviving and Thriving with Lung Cancer
By Brittney Nichols, MPH, RN-BSN, Science and Research Specialist, GO2 for Lung Cancer New treatments, developments, and scientific breakthroughs are always one of the most exciting aspects of the annual American Society of Clinical Oncology (ASCO) conference, but this year they weren't the only highlight. People with lung cancer, [...]
ASCO 2023: Progress for Metastatic (Stage 4) Non-Small Cell Lung Cancer
By Andrew Ciupek, PhD, Associate Director, Clinical Research, GO2 for Lung Cancer New research on metastatic (stage 4) non-small cell lung cancer (NSCLC) was a hot topic at the 2023 American Society of Clinical Oncology (ASCO) meeting. Data from several studies was shared, including new developments for both people [...]
ASCO 2023: Advances in Early-Stage Lung Cancer Research
By Jennifer C. King, PhD, Chief Scientific Officer, GO2 for Lung Cancer While the past decade has seen the development of many new therapies for lung cancer, these drugs are typically first approved for metastatic cancer (cancer that has spread) and not used for earlier stages. We are now [...]
ASCO 2023: New Advances in Lung Cancer Screening
By Heather Law, MA, Associate Director, GO2’s Lung Cancer Registry Lung cancer screening is unavailable for many people at risk for lung cancer. At the 2023 American Society of Clinical Oncology (ASCO) meeting, early results from groundbreaking studies offered hope for expanded screening in the United States. Current [...]
Hope and Time: Tamara’s Story
By Tamara Gabrielli, MD Dr. Gabrielli recently retired after a 30-year career as an anesthesiologist in Maryland and Washington, D.C. She lives in Rockville, MD and has three daughters in their 20s. She just celebrated her 64th birthday. My diagnosis I was diagnosed with lung cancer about 16 [...]
Ask the Experts: Neuroendocrine Tumors
Question: I was diagnosed with small cell lung cancer and my oncologist called it a neuroendocrine tumor. What does that mean? Answer: Neuroendocrine cells are like nerve cells, but they also release hormones into the body like cells of the endocrine system. A neuroendocrine tumor (NET) is a type [...]
Living Room Show Notes: Special Edition – Early Detection, Treatment, and Access to Care
In a Special Edition Lung Cancer Living Room that aired on June 6, 2023, Dr. Steven Liu from Georgetown University shared an introduction to lung cancer, plus an overview of its history including staging diagnosis. In clear, easy-to-understand language, he detailed the most up-to-date information about treatment options, including [...]
My Experience with Brain Radiation
By Shelly Engfer-Triebenbach I was just finishing up my 18th year of teaching music when I noticed myself getting short of breath doing easy things. I went to see my primary care doctor who gave me antibiotics and an inhaler so I could treat what they thought was [...]
Ask the Experts: Questions to Ask Your Doctor
Question: My mom was just diagnosed with lung cancer. What questions should we be prepared to ask at her next doctor’s appointment? Answer: Receiving a lung cancer diagnosis and learning about treatment options can be scary and stressful for a person with lung cancer and their loved ones. [...]
Living Room Show Notes: Clinical Trials and Innovative Trial Design
Clinical trials are a critical part of developing future treatments for lung cancer and other diseases, but low enrollment indicates that they are also often misunderstood by people who might benefit from them. In June’s Lung Cancer Living Room, GO2 for Lung Cancer’s Chief Patient Officer, Danielle Hicks, was [...]
Legislation Introduced to Accelerate Life-Saving Change for Women Impacted by Lung Cancer
The Women and Lung Cancer Research and Preventive Service Act Will Expand Resources to Understand the Science of Lung Cancer in Women San Carlos, CA and Washington, DC – Today, GO2 for Lung Cancer (GO2) applauded the efforts of a bipartisan and bicameral group of lawmakers to address the [...]
Finding Your Calm
Author: Dr. Jennifer K. Renshaw, OTD, OTR/L, OTA/L Have you ever experienced a racing heart, sweaty palms, sleeplessness, worry, headaches, nervousness, decreased focus, or even full body anxiety? As you learn to live with lung cancer and manage all that it can bring, fear of the unknown can overwhelm [...]
Meet Triage Cancer
GO2 for Lung Cancer is proud to partner with Triage Cancer, a national nonprofit organization that provides free education on the legal and practical issues that impact many people who are diagnosed with cancer and their caregivers. Triage Cancer was founded in 2012 by two cancer rights attorneys who [...]
Ask the Experts: Palliative Care Versus Hospice Care
Question: My doctor recently suggested that I start palliative care to help with some of the side effects I’m experiencing from my lung cancer treatment. Is this the same thing as hospice care? Answer: No. Palliative care and hospice care share some similarities, but they are not the same [...]
Living Room Show Notes: Palliative Care, Treating the Whole Person
Palliative care is an important part of many people’s lung cancer care plan. The purpose of palliative care is to improve quality of life. It may provide relief from pain and other symptoms and side effects of lung cancer and is appropriate for patients at every stage of their [...]
A Young Father’s Story
Stephen Huff was just 29 years old and finishing up his first year as a teacher when he started experiencing some changes to his health. His career playing college and professional baseball had recently ended and he thought the weight gain and shortness of breath he was experiencing were [...]
Ask the Experts: The Importance of Multidisciplinary Care Teams
Question: I keep hearing about the importance of having a multidisciplinary care team for my lung cancer treatments. What does “multidisciplinary care team” mean and who might be on it? Answer: A “multidisciplinary care team” is a group of doctors, nurses, and other healthcare professionals from different fields who [...]
Remembering Lung Cancer Champion Win Boerckel
By GO2 Senior Director, Support Initiatives, Maureen Rigney, LICSW Win Boerckel receiving GO2’s inaugural Lung Cancer Support Group Facilitator Award in 2009 Treasured GO2 friend and staunch lung cancer advocate, Winfield Boerckel, died in May following a diagnosis of brain cancer. Some of you may have had [...]
Our 2023 Volunteer Leadership & Advocacy Award Winner: Heidi Nafman-Onda
We are excited to announce that our 2023 Volunteer Leadership & Advocacy Award winner is Heidi Nafman-Onda! This award honors a member of the lung cancer community for their outstanding leadership in volunteerism and advocacy and is awarded annually at GO2’s Lung Cancer Voices Summit. Heidi was a fitness trainer [...]
Ask the Experts: Traveling with Lung Cancer
By lung cancer advocate and experienced world traveler, Elizabeth de Jong Question: I will be traveling this summer for the first time since being diagnosed with lung cancer last year. What should I consider to make travel safe, easy and fun, given my new diagnosis? Elizabeth and Sven [...]
Living Room Show Notes: The Next Wave of Precision Medicine
Treatment for lung cancer has advanced considerably over the years and has been accelerating at an even faster rate this past decade. People diagnosed with lung cancer today have newer, more personalized treatment options that may include targeted therapies, immunotherapies, combination therapies and more traditional chemotherapies or radiation. The [...]
Ask the Experts: What Does NED Mean?
Question: "I have heard people being treated for lung cancer talk about being 'NED.' What exactly does this mean? Is it the same thing as being cured?" Answer: NED stands for “no evidence of disease” and is a term that is like “remission.” NED means that there is no [...]
One Mother’s Story
Lung cancer is the leading cause of cancer-related deaths in women in the United States, taking 171 women’s lives every day. That’s more than breast, ovarian and cervical cancer combined. It affects women of all ages – our grandmothers, aunts, daughters, friends and mothers – so this Mother’s Day [...]
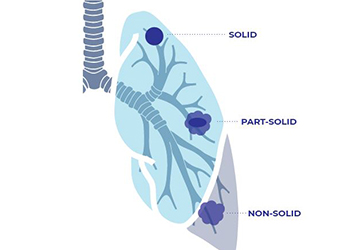
Ask the Experts: Ground Glass Nodules
Question: “I was screened for lung cancer and my scan revealed ground glass nodules in my lungs. What are these and should I be concerned?” Answer: Nodules are abnormal yet common spots that may show up on your lung cancer screening scan or other imaging tests. Some nodules may [...]
Our 2023 Lung Cancer Support Group Facilitator Awardees
GO2's Senior Manager of Support Services, Miranda Goff; award recipients Michelle Hills and Sarah Bechard; GO2's Senior Director of Support Initiatives, Maureen Rigney We are excited to announce our 2023 Lung Cancer Support Group Facilitator awardees are Sarah Bechard and Michelle Hills. Awarded annually since 2009, [...]
The Weight of Shame
Author: Terri Ann DiJulio, lung cancer survivor, CDMRP reviewer, GO2 Summit Planning Committee & NAC Member I am a lung cancer survivor … I am also an outspoken advocate in the lung cancer community. Because of this, many of you may already know that I have been diagnosed with [...]
2023 Lung Cancer Voices Summit Recap
GO2 for Lung Cancer’s Voices Summit is the only annual meeting that brings the lung cancer community together for education, training, connection and advocacy action with our federal government, the largest funder of cancer research. Nearly one hundred advocates from over half of the country gathered in Washington, DC [...]
GO2 for Lung Cancer Announces Two New Board Members
San Carlos, CA and Washington, DC – GO2 for Lung Cancer (GO2), the go-to for one-on-one assistance, supportive connections and treatment information, is pleased to announce that Marsha B. Henderson and Carol Rattray have joined their Board of Directors. “Marsha and Carol are both accomplished women in their fields [...]
Living Room Show Notes: The Vital Patient Role in Advancing Research
An important development in lung cancer research over the past decade or so is the increased prioritization of the patient experience in the research process. Including patient voices in lung cancer research helps to ensure that new research projects best meet the needs of lung cancer patients and survivors. [...]
Ask the Experts: Is Immunotherapy Right for Me?
Question: "I’ve seen commercials for immunotherapy as a treatment for lung cancer. How does it work? How do I know if it’s a good treatment for me?" Answer: Immunotherapy is a type of cancer treatment that helps the body’s own immune system find and attack cancer cells. Your [...]
A Message From Bonnie
Dear Friends, I am reaching out to all of you, my colleagues, my family and my friends to announce that the time has come for me to move on to the next chapter in my life. Planning for and thinking about this next phase has been circling in my [...]
Rays of Hope 2023 Winner: Larry Gershon
We are excited to announce our 2023 Rays of Hope Award winner, Larry Gershon! This award honors the late Richard Heimler. Richard was a GO2 for Lung Cancer board member and a powerful voice of hope in the lung cancer community. Awarded annually, the Rays [...]
U.S. House of Representatives Introduces Bill to Expand Number of Cancers Detected by Screening
The Nancy Gardner Sewell Medicare Multi Cancer Early Detection Screening Coverage Act creates a path forward for Medicare coverage of multi-cancer early detection tests WASHINGTON, D.C. – GO2 for Lung Cancer (GO2) applauds Representatives Jodey Arrington, (R-TX), Terri Sewell (D-AL), Richard Hudson (R-NC), and Raul Ruiz (D-CA) for introducing a [...]
Ask the Experts: Should I Have a Pulmonologist on My Care Team?
Ask the Experts: Should I have a pulmonologist on my lung cancer care team? Yes! The best lung cancer care requires a team of doctors who all specialize in different treatments. This type of team helps you get the best personalized care for your specific type of lung cancer. [...]
We’re Back In-Person!
GO2 for Lung Cancer is excited to announce that after airing our Lung Cancer Living Room monthly events only online for the past three years, we will be returning to in-person Living Room events from our San Carlos office beginning with April’s Living Room on Tuesday, April 18th. We [...]
Meet GO2’s National Ambassador Council
GO2 for Lung Cancer was founded by and for lung cancer patients and survivors. We incorporate the needs and experiences of the lung cancer community into every program we offer and every decision we make. Helping us understand the varied and evolving needs of this community is our National Ambassador [...]
Survivor Spotlight: Tim S.
Tim is a small cell lung cancer (SCLC) survivor, but that’s just one small part of his story. Seven years ago, Tim went to his regular check-up with his primary care doctor and mentioned that he had a cough that wouldn’t go away. He had an x-ray, and a [...]
Living Room Recap: Combatting Lung Cancer Stigma
Unfortunately, "stigma" is something many people diagnosed with lung cancer experience. Lung cancer stigma is usually tied to smoking and may present in many ways. Oftentimes someone newly diagnosed with lung cancer will immediately be asked questions about their smoking history in a very judgmental way, made to feel [...]
Ask the Experts: Talking to Your Doctor About Clinical Trials
Ask the Experts: When should I talk to my doctor about clinical trials? You and your doctor might want to consider joining a clinical trial any time you must decide about your treatment such as at initial diagnosis, upon progression or when a change in treatment is needed for [...]
Celebrating International Women’s Day
Each year, International Women’s Day celebrates the accomplishments of women worldwide while recognizing that change is still needed to make the world an equitable place for all genders. GO2 for Lung Cancer is a women-founded and women-led organization, and one of its important initiatives is to help discover why [...]
Ask the Experts: Prophylactic Cranial Irradiation (PCI)
Ask the Experts: "I have small cell lung cancer and my doctor mentioned the option of having prophylactic cranial irradiation (PCI) to help prevent the cancer from spreading to my brain. I know some people decide to have PCI and others do not. What information do I need to [...]
We Walk This Path Together: Jan Maharam and Irene Stempler‘s Phone Buddy Friendship
GO2 for Lung Cancer's Phone Buddy program began in the early 1990s. Thanks to the many volunteers who give their time to speak with others about their own lung cancer experience, it is still going strong today. The program connects people diagnosed with lung cancer and their loved ones [...]
Clearing the Brain Fog: Eight Ways to Improve Memory and Thinking
Author: Dr. Jennifer K. Renshaw, OTD, OTR/L, OTA/L Certain types of lung cancer treatment may affect your thinking skills and some people notice changes in memory and thought processes during cancer treatment. This doesn’t have to mean a loss of independence. Throughout life we often adjust our way of [...]
Ask the Experts: Second Opinions
Question: I just received a lung cancer diagnosis. I am happy with my oncologist’s plan for treatment, but my son isn’t sure that it’s the right course of action. Is there any harm in getting a second opinion? Answer: When making decisions about lung cancer treatment, getting a second [...]
Living Room Recap: Meet the GO2 Team
GO2 for Lung Cancer relentlessly confronts lung cancer on every front, every day. We were founded by patients and survivors and are dedicated to increasing survival for those at risk, diagnosed and living with lung cancer. Our work focuses on four key goals that we believe will have the [...]
Advocating for Equitable Access to Comprehensive Biomarker Testing
GO2 for Lung Cancer, in partnership with the American Cancer Society Cancer Action Network (ACS CAN) and other health advocacy groups, is supporting a campaign to reduce health disparities by working to ensure equitable access to comprehensive biomarker testing. This campaign aims to improve coverage for and access to [...]
New Study to Understand Why Cancers Progress After Using KRAS Inhibitors
Author: Jennifer C. King, PhD, Chief Scientific Officer GO2 for Lung Cancer has launched the SPARK Study to help understand cancer driven by changes in the KRAS gene. This study is through our medical research consortium, ALCMI (Addario Lung Cancer Medical Institute), in collaboration with Dr. Mark Award and [...]
A Year of Growth for GO2 Research
Authors: Andrew Ciupek, PhD, Associate Director of Clinical Research; Heather Law, MA, Associate Director of Lung Cancer Registry; Shanada Monestime, PharmD, BCOP, Director of Community Engaged Research; Daniel A. Saez, MSc, Manager of LungMATCH Navigation Program 2022 was an exciting year for GO2 for Lung Cancer. We had the privilege [...]
FDA Approvals: New Treatment Landscape in 2023
Authors: Renee Botello, MSc, Treatment and Trial Navigator; Daniel A. Saez, MSc, Manager of LungMATCH Navigation Program In 2022, the treatment landscape for lung cancer continued to evolve to provide breakthrough treatment options for people living with lung cancer. This included targeting mutations that were previously unavailable for patients as [...]
Ask the Experts: Surgery vs. Radiation
Question: I recently received a stage IA lung cancer diagnosis. Is surgery or radiation the best treatment for me? For very early-stage lung cancer, the two most common types of treatment are surgery to remove the cancer and targeted radiation to zap the small spot(s) of cancer. Sometimes when [...]
Lung Cancer Advocates Are Heading Back to Capitol Hill
After three years of virtual advocacy, the 2023 Lung Cancer Voices Summit will be held in Washington, DC, March 19-21. Lung cancer advocates will meet in person with their elected officials to advocate for critical federal research funding on lung cancer. The Lung Cancer Voices Summit is the [...]
Lung Cancer Support Groups: The Pandemic Pivot
Support Groups are a great way to connect with others who understand what you are going through. Studies show that people with lung cancer prefer lung cancer specific groups over those that include people with many types of cancer. GO2 for Lung Cancer created the National Lung Cancer Support [...]
What’s Happening in Research at GO2?
Lung cancer mortality has been declining steadily in the past two decades and the good news is the pace of the improvements have picked up! Last week, the American Cancer Society released their 2022 stats and share that the reduction in mortality has accelerated from 2 to 3% a [...]
Survivor Spotlight: Lori W.
I was diagnosed in June 2021 with stage 4 NSCLC. I did not have any real symptoms until a few days before I went to the emergency room in distress. It took more than a month for my cancer to be typed and staged. I had Non-Small Cell Lung Cancer [...]
FDA Approves Keytruda after Surgery and Chemotherapy for Early Stage Lung Cancers
On January 26, 2023, the U.S. Food and Drug Administration (FDA) approved Keytruda (pembrolizumab) for adjuvant (additional) treatment following surgery and chemotherapy for stage IB, II, or IIIA non-small cell lung cancer (NSCLC). This approval was based on the KEYNOTE-091 clinical trial where people who took Keytruda after surgery [...]
Ask the Experts: Resources for Financial Support
Question: Living with lung cancer is expensive. What resources are available to help pay for day-to-day needs? Answer: Paying for treatment is a concern for most people who are diagnosed with lung cancer. Luckily, there are programs and resources available that can help lessen the financial burden of living [...]
Gone but Not Forgotten: How Kimberly Goodloe Honors Her Sister by Raising Awareness
Kimberly Goodloe didn’t decide to become an advocate - she was called to it after heartache and tragedy struck her life in 2009, and then again in 2022. In 2009, Kimberly received urgent open-heart surgery for an abnormal heart valve. She was born with the defect but was unaware [...]
Living Room Recap: Health Equity – Community Perspectives and Experiences
Inequities in the American healthcare system cause some people with lung cancer to receive worse care than others. Patients who are members of racial, ethnic or sexual minority groups; live in rural areas and have lower income and/or insurance levels are less likely to be screened for lung cancer, [...]
Gathering HOPE is Growing! And You’re Invited!
Have you joined us at a Gathering HOPE event? We first introduced this monthly social/support gathering in Fall 2021 and it has become one of our most popular programs. Dozens of lung cancer community members join us on the 2nd Tuesday of the month at 5-6:30pm PT/8-9:30pm ET for [...]
Ask the Experts: Changing Lung Cancer Diagnosis
Question: My tumor’s original biopsy showed that I had non-small cell lung cancer with an EGFR biomarker. Recently, the cancer started progressing and my most recent biopsy shows that I have transformed small cell lung cancer. What does this mean and how is it treated? When a certain lung [...]
Energy Conservation: Turning ON Your Energy
Author: Dr. Jennifer K. Renshaw, OTD, OTR/L, OTA/L Cancer treatment can make you tired which can make it harder to enjoy leisure activities and complete daily tasks. “Energy conservation” includes tips and tricks for you that use energy in different ways so that you feel better and can do [...]
What a Year! A Message From Our Co-Founders
Dear Friends, As we reflect on this past year, we have much to be grateful for. With the worst of the COVID-19 pandemic behind us, GO2 reaffirmed our commitment to confronting lung cancer on every front, every day to transform survivorship. We reached thousands more patients, survivors, caregivers, and [...]
FDA Approves Krazati for Lung Cancer with a KRAS G12C Mutation
On December 12th, 2022, the Food and Drug Administration (FDA) approved Krazati (adagrasib) for treatment of non-small cell lung cancer (nsclc) where there is a KRAS G12C mutation. This approval is for patients who have received at least one line of prior treatment such as chemotherapy. The FDA based their [...]
Living Room Recap: What is Health Equity and Why Does it Matter?
Heath equity is a popular topic these days, but the concept is more than just a trendy phrase. In general, the health care system in the United States distributes services inefficiently and unevenly across populations which causes some Americans to receive worse care than others. These are called “inequities.” [...]
Ask the Experts: Lung Cancer and Family History
Question: I have a family history of lung cancer – both my mother and grandmother were diagnosed with the disease. Am I at greater risk for lung cancer because of genetics? The role that genetics plays in determining someone’s lung cancer risk is not fully understood. While most [...]
Trends in Lung Cancer Treatments
A popular way to talk about the incredible progress that has been made in lung cancer treatments over the past few years is to share the statistic that more lung cancer treatments have been approved in the past three or five years or than in the previous twenty or [...]
Hundreds of Health Facilities Hosted “Shine A Light on Lung Cancer” Events in November for Lung Cancer Awareness Month
Events brought together patients, health providers and caregivers for hope and healing. SAN CARLOS, CA and WASHINGTON, Nov. 30, 2022 – During November hundreds of health facilities hosted Shine A Light on Lung Cancer events – which made it the largest coordinated lung cancer awareness program in the country. It was the [...]
New Study to Address Cancer Treatment Resistance for People Who Are KRAS Positive
SAN CARLOS, Calif., (November 30, 2022) – The Addario Lung Cancer Medical Institute (ALCMI), a patient-founded not-for-profit global research consortium, has launched the SPARK Study to help researchers better understand treatment resistance in patients with KRAS-positive lung cancer and other cancer types. ALCMI is partnering on the study with [...]
Ask the Experts: Biomarkers and Biopsies
Question: My doctors have determined that my lung cancer is progressing on its current targeted therapy and want to do another biopsy. Why would I need another biopsy since we already know the biomarker type? If I do need another biopsy, should I consider a tissue biopsy or a [...]
What to Expect When you Call GO2’s HelpLine
It can be hard to know where to go with your questions about lung cancer risk, diagnosis and treatment or how to connect with others who have had similar experiences. GO2’s HelpLine staff is here to help! You can contact the HelpLine by phone at 1-800-298-2436 or email us [...]
Survivor Spotlight: Heidi Hanson on Finding Your Strength and Living in the Moment
When Heidi Hanson was diagnosed with stage 4 metastatic small cell lung cancer (SCLC) in December 2021, it wasn’t the typical story. Heidi had been suffering from headaches and dizziness which sent her to the emergency room. It was there that she was told surgery was needed to remove [...]
GO2 for Lung Cancer Honors Champions of the Lung Cancer Community at Simply the Best XVII Gala
FOR IMMEDIATE RELEASE November 14, 2022 Contact: Julia Spiess Lewis julia@perrycom.com 916-601-8282 GO2 for Lung Cancer Honors Champions of the Lung Cancer Community at Simply the Best XVII Gala SAN CARLOS, Calif. and WASHINGTON (November 14, [...]
FDA Approves Imjudo and Imfinzi in Combination with Chemotherapy for Patients with NSCLC
On November 10th, 2022, the Food and Drug Administration (FDA) approved Imjudo (tremelimumab) in combination with Imfinzi (durvalumab) and platinum-based chemotherapy for treatment of non-small cell lung cancer where there are no changes in ALK or EGFR. The FDA based their approval on the results of the clinical trial [...]
Matt Peterson Spotlight Story – Part 2
In 2019, Matt Peterson, a Vietnam veteran, was diagnosed with extensive-stage small cell lung cancer. He was told by his doctor, “This is a life-limiting diagnosis. You will not survive this.” Many people with small cell hear similar words or read information online that feels dire or hopeless, and [...]
Living Room Show Notes – Understanding Lung Nodules
Author: Danielle Hicks, Chief Patient Officer Discovering that you have a lung nodule can be understandably scary. While they are common and can lead to lung cancer, most are not cancerous. According to October’s Living Room speaker Dr. Anthony Lanfranco from the University of Pennsylvania, even those that do [...]
Ask the Experts: LCAM Edition
Question: I was just diagnosed with lung cancer this year, so this is my first Lung Cancer Awareness Month. I want to participate, but I’m not sure how. What are the best ways to support Lung Cancer Awareness Month this year? Participating in Lung Cancer Awareness Month activities is [...]
FDA Approves Libtayo in Combination with Chemotherapy for Patients with NSCLC Regardless of PD-L1 Status
On November 8th, 2022, the Food and Drug Administration (FDA) approved Libtayo (cemiplimab-rwlc) for treatment of non-small cell lung cancer where there are no changes in ALK, EGFR or ROS1 in combination with platinum doublet chemotherapy. The FDA based their approval on the results of the clinical trial Study [...]
The WHAM Report: Investing Just $40 Million New Dollars in Lung Cancer Research Related to Women Has Dramatic Impact on U.S. Economy
Lung cancer kills more women in the U.S. than breast, ovarian and cervical cancers combined. The report commissioned by Women’s Health Access Matters (WHAM) is based on novel microsimulation modeling from the RAND Corporation and documentation consistent with previous analyses on women and Alzheimer’s, coronary artery disease and rheumatoid [...]
GO2 for Lung Cancer Honors Key Partner in Fight Against Lung Cancer
SAN CARLOS, Calif. and WASHINGTON – GO2 for Lung Cancer (GO2 for Lung Cancer) announced it will honor Daiichi Sankyo with the “Simply the Best Award” for their commitment to advancing lung cancer oncology discovery to meet the needs of all patients. The company will accept the award at the Simply [...]
Presidential Proclamation Designates November as National Lung Cancer Awareness Month
As we come together to commemorate Lung Cancer Awareness Month, we are excited to share that President and First Lady Biden who just issued a Presidential Proclamation designating November 2022 as National Lung Cancer Awareness Month! GO2 is honored to have partnered with the White House in developing this [...]
Advances in Precision Medicine for Late Stage Lung Cancer
Authors: Renee Botello, MSc., Treatment and Trials Navigator; Andrew Ciupek, PhD, Associate Director, Clinical Research; Chief Scientific Officer Jennifer C. King, PhD; Shanada Monestime, PharmD, BCOP, Director, Community Engaged Research Every person’s cancer is different, which is why we cannot use a one-size-fits-all approach to treatment. Precision medicine uses [...]
GO2 for Lung Cancer’s Presence at The World Conference on Lung Cancer 2022
Author: Brittney Nichols, MPH, BSN-RN, Specialist, Science and Research This summer, the World Conference on Lung Cancer convened in Vienna, Austria. GO2 shared our research with this community as both lead authors and collaborators on several projects to help advance the diagnosis, treatment, and survival of people with lung [...]
Advances in Treatment for Early Stage Non-Small Cell Lung Cancer
Author: Daniel A. Saez, MSc, Manager, LungMATCH Navigation Program For many years, the treatment options given to patients diagnosed with early stage non-small cell lung cancer (NSCLC) was limited to surgery with or without chemotherapy to improve outcomes. However, like with metastatic NSCLC, the treatment landscape for patients with [...]
Jennifer Moran on Finding Inspiration – and Inspiring Others
Participating in research offers her hope of changing the course of lung cancer Jennifer Moran is married to her high school sweetheart. They have two teenage children in college. A photography major who once dreamed of working at National Geographic, she fell into “a wonderful career in ophthalmic photography.” [...]
GO2 for Lung Cancer’s Kathy Levy a Finalist for Prestigious Catalyst for Equity Award
We’re delighted to announce that Kathy Levy, GO2 for Lung Cancer’s ALCASE Project Manager, was a finalist for the prestigious C2 Catalyst for Equity award. The award recognizes people who have worked to overcome longstanding racial and ethnic disparities in cancer care, ensuring that all people have equitable access [...]
Living Room Recap – Options and Next Steps If Your Lung Cancer Becomes Resistant to Your Course of Treatment
What happens when your cancer becomes resistant to your course of treatment and progresses? That’s the question we explored in the September episode of our Lung Cancer Living Room series. Dr. David Gandara, professor emeritus and co-director of UC Davis Comprehensive Cancer Center’s Center for Experimental Therapeutics, discussed a [...]
Juanita Segura is Inspiring Hope in the Lung Cancer Community
Juanita Segura is a ray of hope in the fight against lung cancer. As a result, she has been awarded the 2022 Rays of Hope Award for her leadership and for inspiring hope as we work together as a community to conquer lung cancer. Upon receiving the award on [...]
Highlights from the World Conference on Lung Cancer
Authors: Andrew Ciupek, PhD, Associate Director, Clinical Research; Jennifer C. King, PhD, Chief Scientific Officer; Daniel A. Saez, MSc, Manager, LungMATCH Navigation Program Doctors and scientists from around the world who are passionate about lung cancer convened last month to bring us the latest on the treatment landscape. GO2 [...]
GO2 for Lung Cancer Receives Grant Award from Disabled Veterans National Foundation
Media Contact: Lori Millner Chief Marketing Officer lmillner@go2.org FOR IMMEDIATE RELEASE: Washington D.C. – September 15, 2022 GO2 for Lung Cancer is honored to announce it received a $25,000 grant from the Disabled Veterans National Foundation (DVNF). The grant will help fund its “Partners and Progress” program which works [...]
Living Room Recap – Reasons to be Optimistic About Small Cell Lung Cancer (SCLC)
Small cell lung cancer (SCLC) can be a highly aggressive form of lung cancer and can be difficult to treat. It is the rarest of the major lung cancer types and represents just 15% of all diagnoses. Unfortunately, few meaningful treatment advancements or improvements in survival rates have occurred [...]
Driving Change: Golf, Community and the Lung Cancer Cause
Curt Groebner is as passionate about golf as he is about fighting cancer. So passionate that for the last 12 years he has held a 24-hour golf marathon that has raised $88,982 for GO2. How a personal connection turned into an ongoing commitment “In our fourth year [of the [...]
FDA Approves First Targeted Therapy, Enhertu, for the Treatment of NSCLC With an Activating HER2 Mutation
On August 11th, 2022, the Food and Drug Administration (FDA) approved Enhertu (fam- trastuzumab deruxtecan-nxki) for treatment of advanced non-small cell lung cancer (NSCLC) with an activating HER2 mutation for patients who have received one prior line of therapy. The FDA based their approval on the results of the [...]
Victory for Veterans
We are humbled and proud to share that GO2 Senior Director Government Affairs Elridge Proctor, MPA, represented our community at the White House for President Joseph R. Biden’s signing of the “Honoring our PACT Act.” This is the most comprehensive act that will deliver vital care and benefits to [...]
New Survey Breaks the Silence on Women, Lung Cancer and Sexual Health
A groundbreaking new survey, conducted by a multi-disciplinary team in GO2 for Lung Cancer’s Lung Cancer Registry, found that sexual dysfunction is prevalent in women with lung cancer. The results were shared by study lead Dr. Narjust Florez (Duma) with top clinicians, researchers and scientists from across the world on [...]
GO2 for Lung Cancer Honors Dr. Rafael Rosell with the Bonnie J. Addario Lectureship Award
GO2 for Lung Cancer recognized Dr. Rafael Rosell at the 23rd International Lung Cancer Congress for his global contributions to oncology, particularly in the field of non-small-cell lung cancer (San Carlos, CA and Washington, DC) – GO2 for Lung Cancer has presented Dr. Rafael Rosell with the 2022 Bonnie [...]
Targeted Therapy Treatment Updates from ASCO 2022
Author: Andrew Ciupek, PhD, Associate Director, Clinical Research Targeted therapy research was front and center at the 2022 American Society of Clinical Oncology (ASCO) Annual Meeting. Data was presented from several studies detailing new targeted therapies and better understanding of how to treat non-small cell lung cancer (NSCLC) with [...]
New Treatments on the Horizon for Small Cell Lung Cancer
Author: Jennifer C. King, PhD, Chief Scientific Officer People diagnosed with small cell lung cancer (SCLC) have historically had limited treatment options—but researchers are working to change that. At the 2022 American Society of Clinical Oncology (ASCO) Annual Meeting, results from a few key studies were presented for the [...]
Immunotherapy Treatment Updates from ASCO 2022: Combinations, Resistance and More
Author: Daniel A. Saez, MSc, Manager, LungMATCH Navigation Program Many patients with non-small cell lung cancer (NSCLC) rely on a class of treatments called immunotherapy to battle their cancer. The significant difference between immunotherapy and targeted therapy (the other main type of treatment that patients with NSCLC receive) is [...]
Coming Together for Equitable Cancer Care
Author: Daniel A. Saez, MSc, Manager, LungMATCH Navigation Program One of the priorities of GO2 for Lung Cancer, alongside many other ally organizations, is to ensure that people diagnosed with lung cancer receive optimal care regardless of their background, socioeconomic status, race or ethnicity. Collectively, we’re working to break [...]
Treatments Beyond Surgery: Early-stage Options from ASCO 2022
Authors: Andrew Ciupek, PhD, Associate Director, Clinical Research and Daniel A. Saez, MSc, Manager, LungMATCH Navigation Program People who are diagnosed with early stage (stage 1-2) non-small cell lung cancer (NSCLC) are generally broken down into two groups: those who can receive surgery to remove their tumor(s) and those [...]
Key Lung Cancer Takeaways from ASCO 2022
GO2 for Lung Cancer team pictured: Joelle Fathi, DNP, RN, ARNP, FAAN; Daniel Saez, MSc; Jennifer C. King, PhD By Jennifer C. King, PhD, Chief Scientific Officer and Daniel Saez, MSc, Manager, LungMATCH Navigation Program The American Society of Clinical Oncology (ASCO) Annual Meeting always features [...]
A Caregiver’s Evolution: From Shock to Engaged Advocate
By Kent Smith, lung cancer caregiver, advocate and GO2 for Lung Cancer Phone Buddy When my wife Debra was diagnosed with lung cancer in 2018, we were in shock. She had been having issues with her hip, which, after several weeks of physical therapy, didn’t resolve. An MRI revealed [...]
Four Takeaways from the 2022 Lung Cancer Voices Summit
Lung cancer advocates from across the country—and world—gathered virtually on May 16-17th at GO2 for Lung Cancer’s annual Lung Cancer Voices Summit. Participants learned about the latest research and treatment developments from experts in the field, received professional advocacy training and met with elected leaders to tell them lung [...]
Survivor Spotlight: Lindy A.
My name is Lindy Arman and I was diagnosed with small cell lung cancer (SCLC) in April 2020. I had recently had knee surgery which resulted in leg swelling that landed me in the emergency room. While there, they performed bloodwork which revealed extremely low sodium levels—which I now [...]
For Rachel Heimler, Summer Jam is a Family Affair
Summer Jam falls on Father’s Day weekend, which is just fine with Rachel Heimler. The first-grade teacher from Manhattan participates in honor of her father, Richard, who died of lung cancer in 2017. Running is Heimler’s jam, something she started doing during COVID to get outside and get some [...]
Tell Congress it’s Personal! Advocacy Day of Action is May 17th
These simple tools make sharing your lung cancer story easy and effective. On May 17th, hundreds of advocates from across the country will meet virtually with elected officials to advocate for the lung cancer community during the 2022 Lung Cancer Voices Summit. Whether you’re participating in the event or [...]
Statement on the passing of Norman Mineta GO2 for Lung Cancer Board Emeritus, Lung Cancer Survivor, former Transportation Secretary and Cabinet Member for Bill Clinton and George W. Bush
Our hearts are heavy as we mourn the passing of Norm Mineta, an extraordinary man, public servant and lung cancer advocate. As an Asian-American he overcame hardships and prejudices early in life to go on to serve at the highest levels of government. Beyond his long and respected career [...]
Small Cell Lung Cancer Transformation: What Is It and How Do We Treat It?
Author: Daniel A. Saez, MSc, Manager, LungMATCH Navigation Program, GO2 for Lung Cancer People with lung cancer receiving treatment may encounter acquired drug resistance—a reduction in treatment effectiveness over time. Tagrisso (osimertinib) is currently a frontline treatment for people diagnosed with non-small cell lung cancer (NSCLC) with an EGFR [...]
Immune Checkpoint Inhibitor Resistance: What To Do Next?
Author: Daniel A. Saez, MSc, Manager, LungMATCH Navigation Program, GO2 for Lung Cancer Immunotherapy, a type of treatment that helps the body’s own immune system fight cancer, is often discussed as a first line of treatment for people with metastatic non-small cell lung cancer (mNSCLC) without an actionable biomarker, [...]
New Screening Guidelines: How They Affect You and What You Need To Know
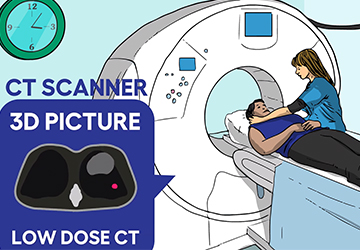
Authors: Andrew Ciupek, PhD, Associate Director, Clinical Research, GO2 for Lung Cancer; Anita K. McGlothlin, Senior Director, Economics & Health Policy, GO2 for Lung Cancer; Angela Criswell, MA, CPS, Director, Quality Screening and Program Initiatives, GO2 for Lung Cancer Lung cancer screening using low dose computed tomography (LDCT) is [...]
Considering Caregivers: The Lung Cancer Registry’s Newest Initiative
Authors: John Wells, MBA, Senior Specialist, Lung Cancer Registry; Jamie L. Studts, PhD, Professor of Medical Oncology and Scientific Director of Behavioral Oncology, University of Colorado School of Medicine; GO2 for Lung Cancer Scientific Leadership Board Member; International Caregiver Survey Working Group; Daniel A. Saez, MSc, Manager, LungMATCH Navigation Program, [...]
Young People Get Lung Cancer Too. We Need to Know Why.
“It took a total of about eight months, four or five doctors visits and multiple X-rays to eventually get that stage IV diagnosis.” -Stephen Huff, lung cancer survivor diagnosed at age 28 When you think about diseases impacting young people, lung cancer might not be at the top of [...]
Meet John: Behind the Scenes of the Lung Cancer Registry
John Wells, MBA-HA is the senior specialist on GO2 for Lung Cancer’s Lung Cancer Registry team. We sat down with John to talk about his role with the Registry, his experience as a health care professional and his advice for people living with lung cancer and their loved ones. My [...]
Survivor Spotlight: Marianne G.
My name is Marianne Gordon. In 2008, at 41 years old, I was rushed to the emergency room after passing out due to a collapsed lung. I had been seeing my doctor prior to this because my blood pressure kept mysteriously dropping which was attributed to some sort of [...]
Breaking Barriers to Advance Inclusive Research
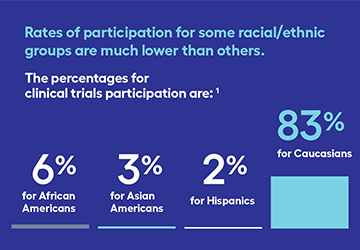
Clinical trials can help anyone—but did you know not all groups have equal representation? A clinical trial is a research study involving people to determine the effectiveness of a new therapy, or combination of therapies. Clinical trials offer people with lung cancer treatment options that might not otherwise be [...]
What Happens in Washington Sends Ripples Across the Land
Your participation in the Lung Cancer Voices Summit also impacts your local community. Sharing your story is an important way to help lawmakers and their staff to understand what it’s like to live with lung cancer—and why we need more research, more screening and more treatment options. That’s why [...]
The Power of Every Voice: Three Lung Cancer Advocates Share Advice for First-Timers
Lung cancer advocacy is about taking action and using your voice to drive lifesaving change. The best part? Anyone can do it. You don’t need to live in Washington, DC or know the ins and outs of Capitol Hill to speak up. Our annual Lung [...]
For Dr. Karen Arscott, “Lung Cancer Will Not Take My Present”
Dr. Karen Arscott has practiced medicine in a variety of venues, most recently caring for patients with substance use disorder. She’s also a two-time lung cancer survivor, a vocal advocate and a member of GO2 for Lung Cancer’s National Ambassador Council. Not initially [...]
Oncology Social Worker Brings Passion and Patients to the Lung Cancer Voices Summit
Kerri Susko, LISW-CP, OSW-C, MBCT Certified, was recruited to move to Prisma Health in Greenville, SC, when she joined the start-up team for the Center for Integrative Oncology and Survivorship. She provides individual psychotherapy to patients and family members. She’s also the Director of the Cancer Support Community at [...]
Walking Side by Side: A Call for Caregiver Support
By GO2 for Lung Cancer with Jamie L. Studts, PhD, Professor of Medical Oncology and Scientific Director of Behavioral Oncology, University of Colorado School of Medicine; GO2 for Lung Cancer Scientific Leadership Board Member; International Caregiver Survey Working Group Caregivers for patients with lung cancer offer vital support [...]
Expanding Access to Lung Cancer Screening: Top 3 Things You Should Know
Authors: Anita K. McGlothlin, Senior Director, Economics & Health Policy, GO2 for Lung Cancer and Angela Criswell, MA, CPS, Director, Quality Screening and Program Initiatives, GO2 for Lung Cancer Lung cancer screening with a low-dose computed tomography (also known as low-dose CT or LDCT) scan is the only current, [...]
Leaving a Legacy
A Secret Service agent based in Phoenix, AZ, Rodney Wellman was diagnosed with stage IV lung cancer in October 2018. He was an avid runner, and decided to use his passion as a way to fundraise for lung cancer research. His first race was a 5K organized by GO2 [...]
Advocacy is Personal. This is My Story.
Author: Elridge D. Proctor, MPA, Senior Director, Government Affairs, GO2 for Lung Cancer Following my graduation from Bethune Cookman College (now Bethune University), a private historical black college founded by educator, humanitarian and civil rights activist Dr. Mary McLeod Bethune, with a degree in [...]
Additional $20 Million for Lung Cancer Research Program
Last week, Congress passed the FY22 Omnibus and on Tuesday, President Joe Biden signed into law the Omnibus spending package that includes an additional $20 million in new funding for the Lung Cancer Research Program. This brings total funding for the Lung Cancer Research Program to $195.5M from FY09-FY22. [...]
FDA Approves New Combination Treatment Before Surgery for Early-Stage NSCLC
On March 4th, 2022, the Food and Drug Administration (FDA) approved Opdivo (nivolumab) for treatment before surgery in combination with platinum-based chemotherapy in patients with stage IB to IIIA non-small cell lung cancer (NSCLC). The approval was based on the results of the clinical trial CHECKMATE-816 which showed that [...]
Everyone’s an Advocate—Including You!
Advocacy is any action that supports, defends or pleads on behalf of a cause or others. It’s a simple word but a powerful concept—knowing that your voice can drive change and save lives. Yet asking people to engage in advocacy causes some people to think it’s too far outside [...]
The Lung Cancer Registry Launches New International Survey Focused on Experiences and Mental Health of Caregivers
Through the first step of information collection, GO2 for Lung Cancer attempts to provide real tailored support programs to the caregivers of people around the world living with lung cancer SAN CARLOS, Calif. and WASHINGTON, D.C., March 1, 2022 – In an attempt to bolster support for caregivers in [...]
Meet the Faces Behind the GO2 for Lung Cancer HelpLine
GO2 for Lung Cancer’s HelpLine team members work hard to provide personalized support and resources to the lung cancer community. But do you know the people behind the voices? Meet two of our wonderful HelpLine team members. Michele Zeh, Manager of Patient Services My name is Michele. [...]
Why We Participate in the Lung Cancer Voices Summit
Elizabeth and Sven de Jong discuss coming together with other advocates with a common purpose. After Elizabeth de Jong was diagnosed with stage IV ALK+ non-small cell lung cancer in September 2016, she and her husband Sven set out to embrace the advice given [...]
New and Improved Lung Cancer Registry
First and Only Global Lung Cancer Registry Through its Lung Cancer Registry, GO2 for Lung Cancer securely gathers information from lung cancer patients across the globe - providing researchers with rich data from which to identify trends that can lead to new and better treatments for lung cancer patients. [...]
A Powerlifter Turned Oncology Nurse Practitioner and a Marketing Pro Who Wanted to Run Away with the Band
Meet Joelle Fathi, DNP, and Lori Millner, two women poised to help GO2 for Lung Cancer and the lung cancer community as we expand our activities and work together to double survivorship rates by 2025. Meet Joelle Fathi, Powerlifter Turned Oncology Nurse Practitioner As GO2 for Lung Cancer's new [...]
New Medicare Lung Cancer Screening Guidelines a Huge Step Forward — More Lives to be Saved
Providers Should Pursue Quality Assurance as Screening Proliferates New Centers for Medicare and Medicaid Services (CMS) recommendations to lower lung cancer screening (LCS) initial age and smoking history requirements can make these exams the most effective cancer screening tests in history. The American College of Radiology® (ACR®), the GO2 [...]
Dan and Brittany: A Love Story in the Time of Cancer
Dan and Brittany Wilson were high school sweethearts. Now, 20 years later, they're married with two young daughters (ages five and seven). When Dan was diagnosed in February 2019 with stage 4A non-small cell Lung cancer, they turned to each other and their families for support. We talked to [...]
Why Can Lung Cancer Happen to Young People?
Our “Lung Cancer Living Room” virtual events portfolio provide the opportunity to learn, question, connect and find support on a wide variety of lung cancer topics. This three-part event series consists of our monthly Lung Cancer Living Room, monthly Gathering HOPE Community Social Hour, and Special Edition Living Room [...]
Fundraiser Spotlight: Luis E. Raez, MD, FACP, FCCP
Luis E. Raez, MD, FACP, FCCP Meet Luis E. Raez, MD, FACP, FCCP, Medical Director & Chief Scientific Officer; Memorial Cancer Institute, Memorial Health Care System; Clinical Professor of Medicine; Herbert Wertheim College of Medicine, Florida International University and GO2 for Lung Cancer Scientific Leadership Board member. [...]
Small Cell Lung Cancer Initiative 2021 Recap and Future Directions
Authors: Amy Kampschroeder MA, OTR/L, CHES, Manager, Patient Outreach and Special Initiatives and Daniel A. Saez, MSc, Manager, LungMATCH Navigation Program In 2021, one of GO2 for Lung Cancer’s priorities continued to be bringing lung cancer education and empowerment to patients to help ensure equitable healthcare. One of the tools GO2 for Lung Cancer used and [...]
Navigating Biomarker Testing Issues
Author: Andrew Ciupek, PhD, Senior Manager of Clinical Research Due to the increasing number of approved targeted therapies for the treatment of lung cancer, clinical guidelines recommend that every patient receive comprehensive biomarker testing. Despite the importance of comprehensive biomarker testing, some people with lung cancer can face barriers [...]
FDA Approvals: New Treatment Landscape in 2022
Author: Daniel A. Saez, MSc, Manager of LungMATCH Navigation Program Despite 2021 being another challenging year, we saw treatment options for lung cancer increase by leaps and bounds. In 2021 the treatment landscape for lung cancer evolved to provide breakthrough treatment options targeting mutations that were previously unavailable for patients. Additionally, [...]
Identification of Chemoradiation Response Mediators in Non‐Small Cell Lung Cancer via Circulating Tumor DNA Analysis
Author: Everett Moding, MD, PhD, Assistant Professor Department of Radiation Oncology, Stanford University School of Medicine A combination of chemotherapy and radiation therapy (chemoradiation therapy) is the backbone of treatment for non-small cell lung cancer (NSCLC) that has spread to the lymph nodes in patients who are not candidates [...]
GO2 for Lung Cancer Hires Lori Millner as Chief Marketing Officer
SAN CARLOS, Calif. and WASHINGTON, January 27, 2022 — GO2 for Lung Cancer (GO2 for Lung Cancer) has hired Lori Millner to serve as Chief Marketing Officer (CMO). In this position, Ms. Millner will oversee global and national marketing efforts to advance GO2 for Lung Cancer’s mission of transforming survivorship by saving, extending, and improving [...]
REGISTRATION OPEN: 2022 Virtual Lung Cancer Voices Summit
Tell Congress It's Personal Registration is now open for the 2022 Virtual Lung Cancer Voices Summit, May 16-17. Don’t miss this opportunity to raise your voice to increase lung cancer research funding! Join advocates from across the country virtually for this inspirational two-day event to [...]
Fundraiser Spotlight: Leah
The 1st Annual Leonard Memorial Golf Tournament was held on Friday, September 17, 2021. The tournament hosted 60 golfers followed by a cookout that included a silent auction and tournament prizes. Over $15,000 was donated in Leonard’s (Len’s) name to GO2 for Lung Cancer. We sat down with Len’s daughter, Leah [...]
Lacing Up for Lungs
Meet Sarah Evans (and her mom Isabelle). In 2021, Sarah teamed up with GO2 for Lung Cancer. As part of the GO2 for Lung Cancer Endurance Team, she raised awareness and much-needed funds to help improve lung cancer survival. We talked to Evans about her passion for running, raising [...]
New Year, New Hope for our Lung Cancer Community
If there is one thing we learned last year, it is that we are stronger together. Despite the ongoing pandemic in 2021, we rallied our community to help transform survivorship. We persevered and expanded vital services to meet the increased demand for information, support, and connection. We launched innovative [...]
Clinical Trials FAQ
By Daniel A. Saez, M.Sc., Treatment and Trials Navigator, GO2 for Lung Cancer Patients often hear the words clinical trial and don’t know what to expect or sometimes even what a clinical trial is. GO2 for Lung Cancer has put together a list of frequently asked questions surrounding clinical [...]
Lung Cancer: No Silly Questions
A lung cancer diagnosis can be physically, mentally, and emotionally draining. One thing you should never worry about is asking your doctor or treatment team questions. You may feel like questions running through your head should be common sense or might be assumed—but rest assured knowing that the only [...]
GO2 for Lung Cancer Launches Improved and Expanded Global Lung Cancer Registry
Registry provides wealth of data to help lung cancer researchers “connect the dots” and empowers patients around the world to be part of uncovering solutions SAN CARLOS, Calif. and WASHINGTON, January 13, 2022 – To further advance lung cancer research by uncovering hidden connections between people with lung cancer [...]
Prioritizing You in 2022
The past year was a challenge for so many, especially those impacted by lung cancer. Now more than ever, it’s important to prioritize YOU and your health and well-being. Going into 2022, here are four worthy commitments to consider. FIND SUPPORT Support in your lung cancer journey is beneficial [...]
GO2 for Lung Cancer and Key Stakeholders Call on Medicare to Expand Lung Cancer Screening Coverage
On March 9, 2021, GO2 for Lung Cancer, American College of Radiology (ACR), and the Society of Thoracic Surgeons (STS) urged the Centers for Medicare and Medicaid Services (CMS) to revise their Medicare coverage policy to reflect the new U.S. Preventive Services Task Force (USPSTF) eligibility thresholds and remove barriers [...]
The Football Bet: A College Fandom Rivalry Leads to Funding for Lung Cancer
It didn't take long for the trash-talking to start. As soon as Jamie Studts logged onto the Zoom call, Nancy Alvey needed a drink of water—from her University of Wisconsin (UW) mug. Studts, of course, had donned his University of Iowa (UI) fleece for the occasion. Meet Nancy Alvey: [...]
Creative Events in 2021: From Scavenger Hunt to Pop-up Drive-in
Creativity might not be the official word of 2021, but the lung cancer community once again showed off its creativity in raising awareness and funds throughout the year. You didn’t have to golf across six states or hike to Mount Everest to participate, though there were of course some [...]
How to Live with Small Cell Lung Cancer: Matt’s Story
My name is Matt Peterson and I am a Vietnam veteran, father, and lung cancer survivor. After a single nodule was found in my lung in 2019, I was diagnosed with small cell lung cancer (SCLC). I was also diagnosed with diabetes. I’ve been through chemotherapy and immunotherapy, but [...]
Finding Hope in a Small Cell Lung Cancer Diagnosis
When you hear the doctor say the words “small cell lung cancer,” life suddenly changes. You may feel overwhelmed and grieved, lost or angry, or you may feel nothing at all. It is okay to move through all kinds of emotions when you are processing a cancer diagnosis. The [...]
FDA Approvals: New Treatment Landscape in 2021
Despite 2021 being another challenging year, we saw treatment options for lung cancer increase by leaps and bounds. In 2021 the treatment landscape for lung cancer evolved to provide breakthrough treatment options targeting mutations that were previously unavailable for patients. Additionally, new immunotherapy options were approved, giving more variety [...]
GO2 for Lung Cancer’s 2021 Centers of Excellence Summit: The Power of Together
Together, we build local excellence to confront lung cancer. GO2 for Lung Cancer’s Center of Excellence Program fosters and promotes the highest quality screening and treatment standards in lung cancer across a national network of local community hospitals. Through COEs, GO2 for Lung Cancer builds the confidence providers and [...]
Erika Hlavacek is Living Her Best Life
In September 2017, Erika Hlavacek was a commercial airline pilot living in the Chicago suburbs with her husband and two children when a cough and progressively worse back pain landed her in the hospital. She was diagnosed with stage 4 lung cancer. Comprehensive biomarker testing soon identified an ALK+ [...]
Improving Access to Comprehensive Biomarker Testing
National advocacy organizations, including GO2 for Lung Cancer, have been advocating for legislative policies to increase and improve access to biomarker testing. Those efforts are increasing, especially at the state level, as legislatures take up biomarker testing bills. Educating lawmakers is critical, as patients face enormous challenges around misdiagnoses [...]
Three Ways to Join the Living Room This Month!
Find empowerment through education, all while connecting with others in the lung cancer community. There is no better time than now to meet with us (virtually) in the Living Room! A few months ago, we introduced our new three-part Living Room series consisting of our monthly Lung Cancer [...]
GO2 for Lung Cancer Hires Joelle Fathi, DNP as Chief Healthcare Delivery Officer
SAN CARLOS, Calif. and WASHINGTON, December 2, 2021 — GO2 for Lung Cancer (GO2 Foundation) has hired Joelle Fathi, DNP to serve as Chief Healthcare Delivery Officer. In this position Fathi will lead GO2 Foundation’s Excellence in Screening and Care department, which includes the GO2 Foundation Centers of Excellence, a network consisting of more than 850 [...]
Senate Passes Resolution Recognizing November as Lung Cancer Awareness Month
On November 30, Senators Marco Rubio (R-FL) and Tina Smith (D-MN) introduced and passed a Senate resolution recognizing November as Lung Cancer Awareness Month. The resolution helps elevate national attention to the disease and encourage more early detection and treatment research to improve outcomes for the millions at risk or [...]
Evy Schiffman on Community and the Power of Storytelling
Evy Schiffman’s husband Neil was diagnosed with stage IV lung cancer in 2011, a month before their 37th wedding anniversary. He passed away four years later. Then in 2018, Schiffman went in for a coronary artery calcium CT scan to detect heart attack and stroke risks. It likely saved [...]
Sydney Barned Says the Stigma Around Lung Cancer Needs to Go
Sydney Barned, MD, was 33 years old and in her medical residency when she was diagnosed with stage 4 ALK-positive lung cancer. A year prior to diagnosis, she woke up with severe shortness of breath and decided she needed a chest X-ray. She was misdiagnosed with pneumonia and put [...]
Survivor Spotlight: A Black Woman’s Experience with Lung Cancer
Hi, I'm Anne Charity Hudley, Ph.D., the North Hall Endowed Chair in the Linguistics of African America and the former Director of Undergraduate Research for the Office of Undergraduate Education at the University of California, Santa Barbara. I study the relationship between language variation and educational practices. I'm also [...]
GO2 for Lung Cancer Honors Champions of the Lung Cancer Community At the Simply the Best XVI Gala
Patients, researchers, physicians, and caregivers honored at annual event SAN CARLOS, Calif. and WASHINGTON, Nov. 15, 2021 /PRNewswire/ -- GO2 for Lung Cancer (GO2 for Lung Cancer) honored leaders in lung cancer research and treatment, as well as dedicated lung cancer caregivers at its Simply the Best XVI Gala held November 13 in San Mateo, CA. [...]
Hank Baskett Wants You to Choose Life Every Day
Hank Baskett was accompanying a friend to an appointment at the VA hospital in Amarillo, TX, when he figured that maybe he should see a doctor about his persistent, hacking cough. An X-ray, an MRI, and a CAT scan followed. Then the doctor told the former U.S. Air Force [...]
Survivor Spotlight: Amy R.
I was diagnosed with stage IV NSCLC adenocarcinoma on November 3, 2017 and found out I was ALK positive on November 26. Being diagnosed with lung cancer came as a total shock, especially since I am a non-smoker. My family, friends, church, and co-workers instantly came alongside me to [...]
Lung Cancer Awareness Month: From Local to Global
The history of lung cancer awareness month (LCAM) exemplifies the famous quote by Margaret Mead that states: “Never doubt that a small group of thoughtful, committed citizens can change the world: indeed, it’s the only thing that ever has.” What started out as an idea from a physician with [...]
Community Keeps JoAnn Berridge Active and Informed
JoAnn Berridge was misdiagnosed, repeatedly. Doctors thought she had allergy-related post-nasal drip; then they thought she had acid reflux. After a year of trial and error and a persistent cough, her primary care physician ordered a chest X-ray in September 2016 and found a 5 cm tumor that had [...]
GO2 for Lung Cancer Honors Key Partner in Fight Against Lung Cancer
Amgen Announced as 2021 "Simply the Best Award" Honoree SAN CARLOS, Calif. and WASHINGTON, November 3, 2021 – GO2 for Lung Cancer announced it will celebrate Amgen with the "Simply the Best Award” at it’s Simply the Best XVI (Under the Stars) Gala on Saturday, November 13. GO2 for [...]
For Sarina Logan, Staying Positive Is About One Day at a Time
Sarina Logan is 41 and was diagnosed with non-small cell lung cancer (NSCLC) in 2018. Thanks to the urging of family friend and GO2 for Lung Cancer Co-founder Bonnie Addario, Logan had comprehensive biomarker testing. The finding that her cancer was EGFR positive (exon 19 deletion) led her to [...]
Treatments and Other Advances in Early-Stage Non-Small Cell Lung Cancer
Author: Rashmi Acharya, MS, Science and Research Specialist, GO2 for Lung Cancer The 2021 World Conference on Lung Cancer (WCLC) had multiple researchers presenting their findings on broad areas of research in early-stage lung cancer. These presentations bring highly encouraging news to the lung cancer community—an interest in understanding [...]
Moving the Needle in Small Cell Lung Cancer
Author: Jennifer C. King, PhD, Chief Scientific Officer, GO2 for Lung Cancer The World Conference on Lung Cancer features research on all types of thoracic cancers—and small cell lung cancer is no exception. There were many talks on new research advances in small cell lung cancer (SCLC). While none [...]
GO2 In Action: Our Involvement in the 2021 World Conference on Lung Cancer
Author: Daniel A. Saez, MSc, Treatment and Trials Navigator, GO2 for Lung Cancer This month, the GO2 for Lung Cancer team virtually attended IASLC’s 2021 World Conference on Lung Cancer. Our science and research team provided strong representation for the organization and the lung cancer community through a combination [...]
A New Look at Targeted Therapies for 2021 and Beyond
Authors: Daniel A. Saez, MSc, Treatment and Trials Navigator, GO2 for Lung Cancer and Jacinta R. Wiens, PhD, Director of Lung Cancer Registry, GO2 for Lung Cancer The World Conference on Lung Cancer (WCLC) highlighted many up-and-coming treatments for patients with different mutations, while also shedding light on proven [...]
A Look at Immunotherapies from the World Conference on Lung Cancer
Author: Andrew Ciupek, PhD, Senior Manager of Clinical Research, GO2 for Lung Cancer The 2021 World Conference on Lung Cancer (WCLC) saw several research presentations that helped increase our understanding of immunotherapy as a treatment for people with lung cancer and how we might better be able to use [...]
Global Lung Cancer Awareness Month Coalition Launches to Transform Survival
Coalition seeks to increase awareness and support for the disease by acting locally, impacting globally. SAN CARLOS, Calif. and WASHINGTON DC, October 28, 2021 – The Global LCAM Coalition (LCAM Coalition), stewarded by GO2 for Lung Cancer (GO2 for Lung Cancer), announced its launch, just in time for Lung [...]
You’re Invited: Simply the Best XVI Under the Stars
Each year, we host our annual fundraising and awards gala in San Carlos, CA. This special evening gives us the opportunity to recognize our industry partners, researchers, doctors, nurses, survivors, and funders, as well as our many individual supporters who share the commitment to ending lung cancer. It is [...]
FDA Approves Tecentriq for Adjuvant Treatment of Non-Small Cell Lung Cancer
On October 15th, 2021, the Food and Drug Administration (FDA) approved Tecentriq (atezolizumab) for treatment following surgery and platinum-based chemotherapy in patients with stage II to IIIA non-small cell lung cancer (NSCLC) whose tumors have PD-L1 expression ≥ 1% as determined by a FDA-approved test. The approval was based on the results of the clinical trial [...]
Ways to Shine A Light on Lung Cancer
Shine a Light on Lung Cancer is the largest coordinated lung cancer awareness program in the United States. Hosted by healthcare facilities during Lung Cancer Awareness Month (November), Shine a Light on Lung Cancer educates, connects, and celebrates our community while raising awareness about the disease. In light of the [...]
The 2021 World Conference on Lung Cancer
Don’t miss our deep dive on topics presented at IASLC's 2021 World Conference on Lung Cancer, including presentations from our very own GO2 for Lung Cancer Science and Research Team. Subscribe to our Magnifying LeNS newsletter and look out for our upcoming special issue on WCLC. By Rashmi [...]
Meet Our New Team Members!
As our community’s needs have grown over the course of the past year, so has our team! Meet the newest members of our GO2 for Lung Cancer staff, working behind the scenes to move the needle for the lung cancer community every day. Alexis Collins, MFA Specialist, [...]
You’re Invited: The New Lung Cancer Living Room
Every month our Lung Cancer Living Room Series brings hope home to people with lung cancer and their families around the world. Anyone who has attended the event, whether in-person or virtually, knows that this is far from your average support group. Started by our co-founder and board chair [...]
How One Cancer Center is Shining a Light on Lung Cancer
UW Health Swedish American is preparing to host a Shine a Light on Lung Cancer event this November. Their goal is twofold: to honor lung cancer patients and caregivers and to provide education for their staff caring for lung cancer patients. “Being a part of a nationally recognized and [...]
A Mother’s Love
Written by Nicole Famularo My mom’s cancer story started in July 2013, although, we came to find out much later that the tumor had probably started to develop years prior to her diagnosis. My parents, my four siblings, and I went to Sandbridge in Virginia Beach, it was our [...]
Survivor Spotlight: Tom A.
I was diagnosed with stage IV adenocarcinoma of the lung on September 7, 2020. There have been many times since being diagnosed that I have said to myself (and others) that I am lucky for my diagnosis. Lucky with a PD-L1 expression of 100%. Lucky that the expression meant [...]
Palliative Care: Improving Quality of Life at All Stages
As many who are faced with lung cancer know too well, the disease and its treatments can cause unwanted symptoms and side effects, both physical and emotional, that can impact quality of life. Lung cancer treatments can cause side effects like fatigue, anxiety, nausea, and pain—not to mention the [...]
FDA Approves Exkivity for Advanced NSCLC with EGFR Exon 20 Insertion Mutation
On September 15th, 2021, the Food and Drug Administration (FDA) approved Exkivity (mobocertinib) for treatment of advanced non-small cell lung cancer (NSCLC) with a change in EGFR called exon 20 insertion. The FDA based their approval on the results of a clinical trial which showed that patients receiving Exkivity [...]
Let’s Help Our Kids Not Have to Worry So Much About Lung Cancer
Michelle Schwarberg grew up in a family of cattle farmers, has 2 kids under 10, and works at her local library. And she has lung cancer. The Kentucky native was diagnosed with the disease at age 34 shortly after the birth of her second child. Short of breath, she [...]
Meet Maureen Rigney, GO2 for Lung Cancer’s Senior Director of Support Initiatives
You’re a licensed social worker. What do social workers do – in general and in your role at GO2 for Lung Cancer? I work here at GO2 for Lung Cancer with our Patient and Support Services team to help people living with or at risk for lung cancer and [...]
Aurora BayCare Medical Center Launches Program to Bridge Gap from Treatment to Survivorship
There’s this quiet that often happens when a cancer patient completes treatment. You go from having your life overwhelmed by doctors, nurses, technicians, and treatments to having nowhere you need to be. Depending on the facility (and the patient), the health care team might ring a bell or otherwise [...]
Treating KRAS G12C Mutated Lung Cancer: A Personal Look
Stage 4 adenocarcinoma non-small cell (aNSCLC) lung cancer treatment has been dictated in recent years by the genetic mutation, or biomarker. Patients received either chemotherapy, immunotherapy, a combination of the two, or a personalized targeted therapy depending on the biomarkers in their cancer. During this period, only a few [...]
From Diagnosis to Determination
Devon Higham had always been active. From the time he could walk there was hardly a day where he did not find himself in the pool or on the soccer field or tennis court. Competitive almost to a fault, he could never placate his insatiable desire to win whether [...]
Antibody Response to COVID-19 Vaccination in People with Lung Cancer
At the World Conference on Lung Cancer, Dr. Jorge Gomez, MD from Icahn School of Medicine at Mount Sinai, New York presented new data on how people with lung cancer respond to vaccination against the SARS-CoV-2 virus that causes COVID-19. The presentation was on behalf of a research consortium [...]
Exciting Breakthrough in Lung Cancer Precision Medicine
For several years stage 4 adenocarcinoma non-small cell (aNSCLC) lung cancer treatment has been dictated by the genetic mutation, called biomarkers, of the patient. A patient’s treatment was seen as either chemotherapy, immunotherapy (or some sort of combination of the two), or a personalized targeted therapy. In the early [...]
The Power of Lung Cancer Research
Activist urges participation to crack the code. Jill Feldman with family Jill Feldman is passionate about advocating for lung cancer research. A mother of 4 young children, she was diagnosed at age 39 with EGFR positive lung cancer. She is the fifth member of her immediate family [...]
Lightening the Financial Burden of Lung Cancer Care
Lung cancer research and treatment options are moving forward faster than ever before. In fact, there have been more advancements for lung cancer in the past two years than over the past four decades, resulting in better patient outcomes. These advancements come with a price tag that pose significant [...]
4 Easy Ways to Unwind
Labor Day Weekend is approaching and there’s never been a better reminder to kick back and do something nice for yourself. When it comes to facing lung cancer, it can be easy to forget how to relax. Taking care of yourself and your needs, whether you are caring for [...]
National Non-Profit Day
This week marked National Non-Profit Day on August 17 to recognize the positive impact of non-profits in the US and communities around the world. People are encouraged to observe this day by learning more about the benefits of supporting a non-profit that often do so much more than fulfill [...]
5 Important Things to Know About COVID-19 and Lung Cancer
It’s no secret that lung cancer survivors are at increased risk for developing severe symptoms and complications from COVID-19. Despite the early summer improvements seen as a result of increased vaccination rates, we are entering a newly difficult and concerning time. As the Delta variant continues its spread, and [...]
GO2 for Lung Cancer Expands Scientific Leadership Board in Effort to Double Five-Year Survival Rate by 2025
SAN CARLOS, Calif. and WASHINGTON, Aug. 5, 2021 /PRNewswire/ -- With the goal of doubling the five-year survival rate of lung cancer patients from 20 percent to 40 percent by 2025, today the GO2 for Lung Cancer (GO2 for Lung Cancer) announced it is expanding its Scientific Leadership Board (SLB). GO2 for Lung Cancer's SLB [...]
GO2 for Lung Cancer to Honor Heather Wakelee, M.D. with the 2021 Bonnie J. Addario Lectureship Award
GO2 for Lung Cancer recognizes Dr. Wakelee, president elect of IASLC, for her leadership on lung cancer clinical trials and research. San Carlos, CA and Washington, D.C., July 30, 2021 – GO2 for Lung Cancer (GO2 for Lung Cancer) will present Heather Wakelee, M.D. with the 2021 Bonnie [...]
Turning the Sound Up on Comprehensive Biomarker Testing
We're turning the sound up on comprehensive biomarker testing. Every person’s cancer is different. Between all of the lung cancer treatment options available, how can you and your treatment team identify the best path for your individual case? Enter: comprehensive biomarker testing. Comprehensive biomarker testing looks for biological changes [...]
Screening Saved My Life
Twelve and a half years ago, I was diagnosed with adenocarcinoma non-small cell lung cancer. I had the lower lobe of my right lung removed on 3/24/09. No cancer cells were found in the lymph nodes, so no follow up treatment was given. My tumor was small and I [...]
Fundraiser Spotlight: Picking Up the Baton and Running Forward
Rod Wellman running the Javelina Jundred At age 52, Dave Wellman is training for his first marathon and fundraising as a member of the GO2 for Lung Cancer’s endurance team at the 2021 TCS NYC Marathon. The inspiration for this undertaking is Dave’s younger brother, Rod [...]
Ensuring Health Equity in Cancer Care
By Jennifer C. King, PhD, Chief Scientific Officer, GO2 for Lung Cancer “Equity: Every Patient. Every Day. Everywhere.” That was the theme for this year’s American Society for Clinical Oncology (ASCO) Annual Meeting. And, indeed, the theme of health equity ran through the meetings tracks and presentations right from the [...]
New Research Unlocks Promise for Treating Small Cell Lung Cancer
By Rashmi Acharya, MS, Coordinator, Science & Research, GO2 for Lung Cancer Unlocking the Biology of SCLC Patients with small cell lung cancer (SCLC) receive one-size-fits-all treatment because of limited knowledge about the biology of the disease. There are currently no actionable biomarkers for SCLC; thus immunotherapy and chemotherapy [...]
Adding New Drugs to Treatment for Early Stage Lung Cancer
By Jennifer C. King, PhD, Chief Scientific Officer, GO2 for Lung Cancer Surgery has been the primary treatment for early stage non-small cell lung cancer (NSCLC). Unfortunately, many people have seen their cancer come back after surgery. Now researchers are exploring whether adding therapies currently used to treat advanced [...]
New Targeted Therapies in the Biomarker Space
By Andrew Ciupek, PhD, Senior Manager, Clinical Research, GO2 for Lung Cancer and Daniel A. Saez, MSc, Navigator, Treatment & Trials, GO2 for Lung CancerNew Hope for Patients with KRASUntil recently, the KRAS mutation was considered un-targetable in most types of cancer, including lung cancer. May 2021 marked the approval [...]
Revisiting EGFR
By Andrew Ciupek, PhD, Senior Manager, Clinical Research, GO2 for Lung CancerA number of studies presented at the American Society of Clinical Oncology’s (ASCO) Annual Meeting related to the treatment of non-small cell lung cancer (NSCLC) with an EGFR mutation.Tagrisso remains an important and effective standard of care option for [...]
Immunotherapy Update
By Rashmi Acharya, MS, Coordinator, Science & Research, GO2 for Lung CancerChemotherapy has been the standard treatment option for Non-Small Cell Lung Cancer (NSCLC) without actionable biomarkers. Recently, immunotherapy treatments have been added into the treatment mix. Several studies presented at the American Society of Clinical Oncology’s Annual Meeting offer [...]
Empowerment Through Education
A lung cancer diagnosis can feel overwhelming, even confusing. And while every diagnosis is different, one thing is the same: if you or someone you love is faced with lung cancer, we’re here to help. GO2 for Lung Cancer is dedicated to providing patients, caregivers, healthcare professionals, and advocates [...]
Helping Health Care Providers Talk about Screening
Screening for lung cancer before symptoms appear is important. Without it, most people don’t see signs of the disease until it has spread to other parts of the body, which is harder to treat. Just ask Barney B. who responded to a screening advertisement, discussed it with his health [...]
Endurance Golf, Speed Golf, and a Passion for Ending Lung Cancer
Curt Groebner is passionate about golf—and fighting cancer. So passionate that he convinced 3 friends to play golf with him, once a year, for 24 hours straight. For 10 years. In the process he raised over $68,000, much of it for GO2 for Lung Cancer. “In our fourth year, [...]
First, the Phone Buddy Program gave her a lifeline.
Wendy (left) with her Phone Buddy, Cecily (right) Then the Phone Buddies became friends. Wendy Williams and Cecily Hatchitt met through GO2 for Lung Cancer’s Phone Buddy Program. Then they became something more: friends. Meet Wendy Williams, a Kennesaw, GA, resident who reached out to GO2 for [...]
Celebrating a Lung Cancer Hero
Susan and Rodney Wellman GO2 for Lung Cancer recently asked people to share stories about their lung cancer heroes—people who have inspired them and gone above and beyond for the cause. Rodney Wellman was a lung cancer hero. A Secret Service agent based in Phoenix, Wellman was [...]
Expanding Our Campaign for Lung Cancer Screening
Lung cancer screening saves lives. We know this. It has been our rallying cry for decades and has remained a core initiative. Now, we’re excited to partner on the "Screen Your Lungs" campaign to help meet our goal of bringing lung cancer screening to the millions at risk for the disease, a [...]
Thank you to Our 2021 Summer Jam Event Chairs
For the second year in a row, we are excited to be hosting our Summer Jam Virtual 5K Your Way on Saturday, June 26. This event brings people together from across the country in celebration of the beginning of summer and to raise awareness and funding for lung cancer. [...]
GO2 for Lung Cancer Named an Official Charity Partner of the 2021 TCS New York City Marathon
GO2 for Lung Cancer was named an Official Charity Partner for the 2021 TCS New York City Marathon, taking place on November 7. This year serves as the 50th celebratory running of the marathon. GO2 for Lung Cancer will again be among the more than 400 official charity partners [...]
U.S. Supreme Court Upholds the Affordable Care Act
Breaking news! Exciting News! Today, the U.S. Supreme Court upheld the Affordable Care Act (ACA) in California v. Texas, a legal challenge that sought to strike down the law. This is a great victory for all patients with chronic or life-threatening illnesses, including our own lung cancer community. Now [...]
Representation in Clinical Trials
Increasing diversity within lung cancer clinical trials is of critical importance for patients, caregivers, and researchers. To make sure that you are informed and prepared to share the message, we’ve pulled together some helpful blog posts and downloadable materials on representation in clinical trials. First—dive into this post on [...]
Alabama Awareness, Screening & Education (ALCASE) Project
Kathy Levy of GO2 for Lung Cancer with family As manager of our ALCASE project, Kathy Levy leads a trailblazing program dedicated to changing health outcomes in seven largely rural, primarily underserved, predominately Black counties in Alabama. ALCASE, a joint project between the GO2 for Lung [...]
Lung Cancer and Black Americans
Lung cancer is the leading cause of cancer death in Black Americans, and, despite a lower incidence rate, five-year survival is lower for Black patients. Review and share the stats in our latest fact sheet on Lung Cancer and Black Americans and join us in supporting National Black Family [...]
Survivor Spotlight: Barney B.
With no symptoms of lung cancer, I went in for a low-dose CT scan after receiving a direct mail flier from my health care provider system, Baylor Health (Dallas, TX). I had quit smoking approximately ten years earlier at age 48 and had a family history of various cancers. [...]
Research into Acquired Drug Resistance in Patients with ALK+ NSCLC
Amanda Nerstad, SPACEWALK participant, with family By Daniel A. Saez, MSc, Treatment and Trials Navigator, GO2 for Lung Cancer One of the biggest concerns within the mutation positive non-small cell lung cancer (NSCLC) community is the concept of acquired resistance to targeted therapies. Acquired resistance is when [...]
Survivorship: Insert your definition here.
Today more than ever before, words matter. And, as a society, we are more sensitive to descriptive preferences. People are living longer, and better, with lung cancer than ever before. It’s time we think about survivorship (or whatever you decide to call it) differently and take our cues from [...]
Comments of Hope
Courage and hope. These critical qualities are what our co-founder and board chair Bonnie J. Addario profiles in a new publication titled The Living Room: A Lung Cancer Community of Courage. Finding hope and moving forward is a key theme that Bonnie probes with her community of Lung Cancer [...]
This Physician is on a Mission to Screen Every High-Risk Kentuckian
Kentucky has a lung cancer problem. Michael Gieske, MD, is on a mission to raise awareness about the disease and detect, treat, and save as many people as possible. A primary care physician, Gieske is the director of lung cancer screening at St. Elizabeth's HealthCare. The Kentucky native [...]
Highlights from ASCO 2021
By Rashmi Acharya, MS; Andrew Ciupek, PhD; Jennifer C. King, PhD; and Daniel Saez, MSc The annual meeting held by the American Society of Clinical Oncology (ASCO) is the stage for presenting many of the most impactful advancements in cancer research – including in lung cancer. Health equity was the major theme of the 2021 meeting, held June 4-8, 2021, and multiple studies showed that there are many disparities in lung cancer care [...]
GO2 for Lung Cancer Expands Early Detection Efforts to Include Incidental Pulmonary Nodules (IPN) to Save More Lives
GO2 for Lung Cancer to launch a webinar series and toolkit on building IPN management programs SAN CARLOS, Calif. and WASHINGTON, D.C., June 10, 2021 – To help diagnose early-stage lung cancer when it is more treatable and even curable, GO2 for Lung Cancer (GO2 for Lung Cancer) is [...]
Landmark Study Underscores Lung Cancer Differences in Young Adults
Young lung cancer illustrated by Stephen H. and family. Data from a landmark research study shows that the biology behind “young” lung cancer – lung cancer diagnosed under the age of 40 – is different from that of the general population. The Genomics of Young Lung Cancer study, [...]
Legislation Introduced for Lung Cancer Screening and Early Detection
We are proud to endorse and provide strong support for Katherine’s Lung Cancer Early Detection and Survival Act of 2021, introduced by our House Congressional Lung Cancer Caucus Chair Representative Brendan Boyle (D-2-PA) and co-champion Senator Tina Smith (D-MN). Recognizing that too many lives are lost to lung cancer [...]
2021 Illinois Bill Passage on Biomarker Testing Coverage
GO2 for Lung Cancer is a proud supporter of the recently passed Illinois State Bill (HB 1779), which requires Medicaid as well as state-regulated private insurance plans to cover comprehensive biomarker testing when supported by medical and scientific evidence. Currently, insurance coverage for biomarker testing is failing to keep [...]
First Targeted Therapy Approval for Non-Small Cell Lung Cancer with KRAS Mutation
On May 28, 2021 the United States Food and Drug Administration (FDA) approved Lumakras (sotorasib) for the first line treatment of advanced non-small cell lung cancer (NSCLC) with tumors having the G12C change in KRAS. The FDA based their approval on the results of the clinical trial CODEBREAK 100 which [...]
Survivor Spotlight: Linda S.
I found out I had lung cancer as I was driving my car to the Women’s March in Washington DC after the 2016 election. At the time, I remember asking the Universe to allow me to live long enough to vote again. I’ve found strength in my family and friends [...]
FDA Approves Rybrevant as First Targeted Treatment for Patients with NSCLC with EGFR Exon 20 Insertion Mutations
On May 21st, 2021, the Food and Drug Administration (FDA) approved Rybrevant (amivantamab-vmjw) for treatment of advanced non-small cell lung cancer (NSCLC) with a change in EGFR called exon 20 insertion. The FDA based their approval on the results of the clinical trial CHRYSALIS which showed that patients receiving Rybrevant [...]
Survivor Spotlight: Lee M.
In January 2017, I was diagnosed with stage IIIB non-small cell lung cancer at age 43. I was blindsided. I had run two half-marathons within the past 6 months and having lung cancer was something I never even considered as a possibility. I was lucky [...]
The Living Room: A Lung Cancer Community of Courage
Breaking down the barriers and stomping out the stigmas associated with lung cancer. San Carlos, CA/Washington D.C. – Bonnie Addario, co-founder and chair of GO2 for Lung Cancer, the largest lung cancer patient advocacy organization in the United States and a survivor herself, profiles 21 men and women battling [...]
Breathe Free Concert Supports Research into Lung Cancer in Young Adults
Emily Bennett Taylor, Stage IV lung cancer survivor, with family There is a growing cohort of younger adults who are being diagnosed with lung cancer. Alarmingly, the research points to an increase not only in diagnosis at a young age—but particularly in women. Upstage Lung Cancer is proud [...]
Spend Your Summer with Us!
You are invited! Help us double-down on survival by participating in one or more fundraising events with GO2 for Lung Cancer this summer. Each event is open to the entire lung cancer community. No matter where you live, you can join in and help fund programs aimed towards doubling [...]
GO2 for Lung Cancer’s Focus on Women and Lung Cancer
May is Women’s Health Month, and GO2 for Lung Cancer has partnered with the Addario Lung Cancer Medical Institute to investigate how and why some young women’s rates of lung cancer are increasing, despite never having smoked. Watch the video below from GO2 for Lung Cancer’s Dr. Andrew Ciupek to learn more. [...]
GO2 for Lung Cancer Seeks to Better Understand Needs of People with Small Cell Lung Cancer
Innovative effort aims to improve outcomes for historically underserved lung cancer patient community. SAN CARLOS, Calif. and WASHINGTON, D.C., May 11, 2021 – GO2 for Lung Cancer (GO2 for Lung Cancer) has launched a landmark initiative to identify the needs of the Small Cell Lung Cancer (SCLC) community. SCLC [...]
A Family Contributes to the Lung Cancer Cause
A generous contribution from the DeCesaris family is helping us double survivorship by 2025 by growing our reach to individuals at risk and patients across the country. When Geaton, patriarch of the family and their “rock” passed from lung cancer, the family began donating to GO2 for Lung Cancer, [...]
Fundraiser Spotlight: Carlos Cares
On December 22, 2019, just one month after his 50th birthday, Carlos J. Perez-Albuerne died after living for two and a half years with incurable lung cancer. As they approached the first anniversary of his death, his close-knit family was looking for a way to honor his memory. [...]
The Impact of Lung Cancer Across Generations: One Family’s Story
Usha Jain (left) and daughter, Amita Jain (right) Lung cancer doesn’t just impact one person—it affects all those who love them. Perhaps no family understands this better than the Jain-Patkar family. Meet Usha Jain, a retired professor in the Department of South and Southeast Asian [...]
National Nurses Day: Celebrating One of Our Own
Today is National Nurses Day, and we’re celebrating one of our own: Kim Parham, RN, BSN, CN-BN, and Director, Quality Care & Clinical Relations, of our Excellence in Screening and Care program. We caught up with Kim to hear a little bit about her history as a nurse and [...]
GO2 for Lung Cancer Statement on Proposed FDA Actions on Menthol Cigarettes
Today, the U.S. Food and Drug Administration (FDA) announced it is working toward issuing proposed rulemaking to ban menthol as a characterizing flavor in cigarettes and ban all characterizing flavors (including menthol) in cigars to reduce disease and death from use of combusted tobacco products in the United States. [...]
Meet Daniel Saez, LungMATCH’s Treatment and Trials Navigator
Daniel A. Saez, M.Sc., Treatment and Trials Navigator Guiding patients to clinical trials and other treatment options. Daniel Saez is the voice of LungMATCH. He’s the person you hear and the navigator you’re most likely to talk to when you call GO2 for Lung Cancer’s HelpLine looking [...]
A More Promising, and Personalized, Future for Small Cell Lung Cancer Patients
Author: Carl M. Gay, MD, PhD of MD Anderson Cancer Center Dr. Carl Gay received our 2018 Young Investigator Award. He graciously agreed to write this summary of new research, including his own, that is poised to make a difference for people with small cell lung cancer. A new [...]
A Call for Major Commercial Payers to Revise Screening Coverage
We recently requested that payers (healthcare insurance companies) expeditiously update their lung cancer screening (LCS) coverage policies in accordance with the recently updated United States Preventive Services (USPSTF) guidelines. In concert with other key stakeholders, letters were sent to the top five national private insurance companies—Aetna, Anthem, Cigna, Health Care Services Corporation, [...]
You’re Invited: Music, a Book, Bonnie, and Young Lung Cancer
Bringing hope to patients with lung cancer has been a trademark of our co-founder and board chair Bonnie J. Addario. A longtime fundraising partner—UpStage Lung Cancer—is supporting several projects championed by Bonnie with a podcast and musical events. We invite you to participate! Learn about an exciting study [...]
Side Effects Myths Busted!
Lung cancer and its treatment are complex. With different treatments come different side effects—and myths. Thanks to our HelpLine team, we’re debunking some of the most common myths about lung cancer treatment side effects. MYTH 1: If you tell your doctor about side effects, they will stop your [...]
A Primer on Small Cell Lung Cancer and the Role of Clinical Trials in Treatment
By Rashmi Acharya, MS, Coordinator, Science & Research, GO2 for Lung Cancer 1. What is Small Cell Lung Cancer? Small cell lung cancer (SCLC) is a type of lung cancer named for the very small, oval shaped cancer cells seen under a microscope. It is a rapidly growing type [...]
Why are Young People Getting Lung Cancer?
GO2 for Lung Cancer and the Addario Lung Cancer Medical Institute (ALCMI) recently launched a new study, Epidemiology of Young Lung Cancer (EoYLC), to better understand why some people under the age of 40 develop lung cancer. Many of them have no history of smoking. The new study [...]
Treatment Insights for KRAS Mutated Non-Small Cell Lung Cancer
By Daniel A. Saez, MSc, Treatment and Trials Navigator, GO2 for Lung Cancer Rebecca (KRAS driven stage 4 NSCLC patient) and Wayne Yeung (caregiver) Since the original publication of this article, the United States Food and Drug Administration (FDA) approved Lumakras (sotorasib) for the first [...]
COVID-19: A Year of Reflection
On March 11, 2020, our lives changed forever when the World Health Organization declared that COVID-19 represented a new global pandemic. One year later, we reflect on where we started and where we are now. First, we pause to honor the lives of the 2.6 million people globally, including [...]
What is Small Cell Lung Cancer?
By Daniel A. Saez, M.Sc., Treatment and Trials Navigator, GO2 for Lung Cancer Patients diagnosed with Small Cell Lung Cancer (SCLC) have new hope because of recent advances in early detection and treatment. To amplify this positive message, we are working with industry partners to empower patients diagnosed with [...]
Five Communities Spring Into Action for GO2 for Lung Cancer
Thank you to everyone who joined us for our first-ever Step into Spring Virtual Step Challenge! You blew our goal out of the water, logging over 10 Million Steps for lung cancer and raising over $91,000 for our programs dedicated to doubling lung cancer survival. Congratulations! You came out [...]
Advocacy for Lung Cancer is Personal
What an impact our community can make! On March 23-25, 2021, we brought together survivors, patients, caregivers, family & friends, healthcare professionals to listen, learn, share, and tell their members of Congress about the needs of the lung cancer community and demand action. Look at the numbers: 160 [...]
Rays of Hope 2021 Winner: Terri Ann DiJulio
Rays of Hope Awardee Terri Ann DiJulio We are excited to announce our 2021 winner of the Rays of Hope Award—Terri Ann DiJulio. This award—bestowed on an individual that has demonstrated leadership and inspired hope— honors the late Richard Heimler, a past Lung Cancer Alliance board member [...]
Toxic Exposure in the American Military
We are proud to endorse the Toxic Exposure in the American Military (TEAM) Act reintroduced this week by Senators Thom Tillis (R-NC) and Maggie Hassan (D-NH), and in the House by Representative Mike Bost (R-IL) which will expand health care eligibility and create a framework for establishing presumptive service connection for [...]
Survivor Spotlight: Lisa H.
I was diagnosed almost six years ago (3/27/15) with Stage IV NSCLC adenocarcinoma, KRAS G12V and am currently NED. I’ve now become more involved in patient advocacy and support for newly diagnosed lung cancer patients, and participated in the 2021 Lung Cancer Voices Summit. My lung cancer diagnosis was [...]
Fundraiser Spotlight: Irina
Grit and Determination Fueled This Fundraiser Irina Lucaciu recently tackled an incredible athletic feat. In early March, she challenged herself to run four miles every four hours over the course of 48 hours. She ran during the day and at night; in calm weather [...]
Physician and Patient Groups Call On CMS to Update Medicare Lung Cancer Screening Coverage
New Medicare Coverage Should Reflect USPSTF Eligibility Thresholds and Reduce Barriers to Care Washington (March 18, 2021) -- Medicare lung cancer screening coverage should be updated to reflect new U.S. Preventive Services Task Force (USPSTF) eligibility thresholds and remove barriers that keep at-risk populations from accessing these lifesaving exams. [...]
Update: Multi-Cancer Screening Coverage
We are proud to provide our strong support for the Medicare Multi-Cancer Screening Coverage Act of 2021 reintroduced March 16, 2021 by U.S. Reps. Terri Sewell (AL-07), Jodey Arrington (TX-19), Raul Ruiz (CA-36), and Richard Hudson (NC-08) to ensure that as science develops new methods of finding cancers at [...]
A Very Personal St. Patrick’s Day
March 17, 2004, St. Patrick’s Day, became one of the most significant days in our lives. It is the day our mom, against all odds, became cancer free. Bonnie Addario (center) with her daughters Andrea Parks, Chief Development Officer (left) and Danielle Hicks, Chief Patient Officer (right). [...]
Take Action! Email, Tweet, Tell Congress to Support Lung Cancer Research
At the 2021 Lung Cancer Voices Summit, advocates across the lung cancer community will come together to learn, network, and raise awareness about lung cancer and the need to focus funding for research and new treatment options. This year’s theme is Tell Congress It’s Personal. Lung cancer is personal—to [...]
Women and Lung Cancer and Congress in 2021
We gladly mark another step forward in long-standing efforts to elevate lung cancer as a women’s health imperative and accelerate efforts to transform survivorship for our community. Bi-partisan congressional leaders responded to our call to action and reintroduced the Women and Lung Cancer Research and Preventive Services Act of [...]
3 Keys to a More Integrated Approach to Lung Health
McLeod Health was looking for a better way to detect and track lung health across its one million plus patient population. The health system includes 7 hospitals across South Carolina, along with a growing network of primary care doctors and family physicians. McLeod turned to GO2 for Lung [...]
Cracking the Code on Lung Cancer in Younger Adults
Stephen Huff & family, Stage IV lung cancer Why are younger adults getting lung cancer? There is a growing cohort of younger adults who are being diagnosed with lung cancer. Alarmingly, the research points to an increase not only in diagnosis at a young age—but [...]
Jog for Jill—and Honor the Woman Who Inspired a Generation
One person can make a difference. If you have any doubt, look no further than the legacy of Jill Costello, a college student at the University of California at Berkeley who was diagnosed with Stage 4 lung cancer at age 20. Her motto was “Beat Lung Cancer,” a [...]
Meet Catherine Paykin, a Social Worker on a Mission
Catherine Paykin (left) with GO2 for Lung Cancer's Miranda Goff (right). Catherine Paykin, MSSW, LCSW, is on a mission to make sure lung cancer patients are heard and supported. An oncology social worker at NYU Langone Health Perlmutter Cancer Center she meets with lung cancer patients one-on-one, [...]
Life-Saving Screening Expands to Reach More Americans
Today, the United States Preventive Services Task Force (USPSTF) published its final recommendation for low dose CT lung cancer screening, which expands screening recommendations by lowering the age minimum to 50 years and lowering the smoking history to 20-pack years. We commend the USPSTF for bringing life-saving screening to more [...]
At Dignity Health, AZ, Finding Inspiration as a Team
Norton Thoracic Institute at Dignity Health St. Joseph’s Hospital and Medical Center in Phoenix, AZ is taking teamwork on behalf of the lung cancer community to an entirely new level. Dignity Health’s Norton Thoracic Institute has a multidisciplinary program dedicated to catching and diagnosing early lung cancer via [...]
Increasing Diversity in Clinical Trials
It may seem like clinical trials are suddenly top of mind here at GO2 for Lung Cancer, with new articles about inclusive research and demystifying them with a brand-new FAQ. But clinical trials and the diversity of the patients within them have always been a high priority.Jargon-Free Materials and TrustA [...]
Join Us for the Virtual Lung Cancer Voices Summit
Highlights Include a Special Lung Cancer Living Room on COVID-19 and 2 Networking Happy Hours Join us, March 23-24, for the Lung Cancer Voices Summit. There’s still time to register and participate in the educational and networking sessions at this year’s Voices Summit. Join other patients, survivors, caregivers, [...]
FDA Updates Approval for Lobrena to First Line Treatment for Advanced NSCLC Patients with Changes in ALK
On March 3rd, 2021, the Food and Drug Administration (FDA) approved Lobrena (lorlatinib) for the first line treatment of non-small cell lung cancer (NSCLC) which has spread outside of the lungs that has a change in ALK identified by an FDA approved test. The FDA based their approval on [...]
GO2 for Lung Cancer Leads Study to Improve Participation of Black Communities in Lung Cancer Clinical Trials
SAN CARLOS, Calif. and WASHINGTON – GO2 for Lung Cancer (GO2 for Lung Cancer) announced its leadership in the launch of an important multi-institutional study to determine how to improve the participation of Black communities in lung cancer clinical trials. While participation in clinical trials by all cancer patients in the [...]
FDA approves new immunotherapy, Libtayo, for patients with metastatic NSCLC and high PD-L1
On February 22nd, 2021, the Food and Drug Administration (FDA) approved Libtayo (cemiplimab-rwlc) for the treatment of advanced non-small cell lung cancer (NSCLC) with no changes in EGFR, ALK, or ROS1 but with high PD-L1. The FDA based their approval on the results of a clinical trial which found [...]
New Study Seeks Origins of Young Lung Cancer
Addario Lung Cancer Medical Institute and GO2 for Lung Cancer to learn about environmental, lifestyle, and demographic risks of developing lung cancer under the age of 40 San Carlos, CA/Washington DC – Continuing their collaboration to transform lung cancer survival and to accelerate novel research advancements for people with [...]
GO2 for Lung Cancer Advocates for COVID-19 Vaccine Prioritization
Today, GO2 for Lung Cancer joined a broad spectrum of organizations representing laboratory, translational, and clinical researchers; other health care professionals; patients with cancer; and patient advocates in sending a letter to the Biden administration and other public health officials that highlights the importance of prioritizing patients with active [...]
5 Reasons to Attend the Voices Summit
Register by February 19 to participate in meetings with your Members of Congress! Our annual Voices Summit is inspiring and empowering—but don’t take it from us. Hear from these lung cancer advocates on why they are joining us virtually next month, and why you should too. Every person in [...]
New Therapy for Small Cell Lung Cancer Patients: FDA Approval of COSELA (trilaciclib)
On February 12th, 2021, the Food and Drug Administration (FDA) approved Cosela (trilaciclib) for patients with extensive stage small cell lung cancer (ES-SCLC) (cancer which has spread outside of the lung) to help reduce the frequency of chemotherapy-induced bone marrow suppression in adults receiving certain types of chemotherapy. The [...]
The Race to 60K Before 60
Terri Ann DiJulio set an audacious goal last winter of raising $60,000 for GO2 for Lung Cancer before she turns 60 in January, 2021. The birthday fundraising campaign, entitled 60 for Sixty was off to a strong start... then COVID-19 hit. Many people might have abandoned their goal. But [...]
2020 World Conference on Lung Cancer (in 2021)
By Jennifer C. King, PhD, Chief Scientific Officer, GO2 for Lung Cancer New advances in lung cancer research were presented earlier this year at the 2020 World Conference on Lung Cancer (WCLC). Due to the COVID-19 pandemic, this conference had been rescheduled from August 2020 and was held virtually. [...]
FDA Approves Tepmetko (tepotinib) for NSCLC with MET Exon 14 Skipping Mutations
On February 3rd, 2021, the Food and Drug Administration (FDA) approved Tepmetko (tepotinib) for treatment of advanced non-small cell lung cancer (NSCLC) with a change in MET called exon 14 skipping. The FDA based their approval on the results of the clinical trial VISION which showed that patients had a [...]
Out-of-Pocket Costs for Lung Cancer Patient Care
A cancer diagnosis is emotionally, physically, and in many cases, financially challenging. According to the NCI, “Some cancer survivors report spending more than 20% of their annual income on medical care.” For many with and without insurance, the cost of care can lead to what is known as financial toxicity. [...]
Vaccine Prioritization
Because of the dual impact on our respiratory system, we’ve coined the term Lung Cancer + COVID-19 = Twice the Problem in an earlier blog post. Another strong step has been taken in our comprehensive strategy to elevate the needs of our community by reinforcing that those with lung cancer [...]
Update for Week of Jan 25 to the Joint Statement on COVID-19 From Lung Cancer Advocacy Groups
As of January 22, we stand at 24.8 million COVID-19 cases in the US, with 412,936 deaths. The CDC reports that 15.1 million Americans have received at least one dose of vaccine, with 2.4 million people being fully vaccinated. President Biden and his administration have made addressing the COVID-19 pandemic [...]
1 + 1 = Twice the Problem
We are now several weeks into the new year. The good news is that we now have COVID-19 vaccines approved. The bad news is that the vaccines are not getting to people quick enough, especially to those with lung cancer who are at an increased risk because of co-morbidities.Both lung [...]
Learning About Everyone: The Importance of Advancing Inclusive Research
By Jennifer C. King, Ph.D., Chief Scientific Officer, GO2 for Lung Cancer Are scientists learning enough about how people just like you respond to new treatments? You may be surprised. Clinical trial participation rates among people with cancer in the United States are low, with only about 8% of those [...]
Lung Cancer Registry: Partnering with Patients and Caregivers to Drive Research Forward
By Jacinta R. Wiens, Ph.D., M.S., Director, Lung Cancer Registry, GO2 for Lung Cancer The Lung Cancer Registry (LCR), created by the GO2 for Lung Cancer, is a global online database that was launched in 2016. The LCR was designed so that patients could contribute their individual experiences with lung [...]
When Worlds Collide: Research at the Intersection of COVID-19 and Lung Cancer
By Amy C. Moore, Ph.D., Director, Science & Research, GO2 for Lung Cancer 2020 started off on a high note – we were celebrating the largest single year drop in cancer mortality. Then COVID-19 showed up and the cancer world was put on high alert. Patients with lung cancer [...]
How Biomarker Testing Affects Patients
By Daniel A. Saez, M.Sc., Treatment and Trials Navigator, GO2 for Lung Cancer In today’s lung cancer care landscape, biomarker testing has become a crucial part of the treatment decision for many oncologists. Targeted therapies can be more effective than chemotherapy for patients with driver mutations123 including approved medications [...]
Trends in Treatment of EGFR+ NSCLC after Progression Following Tagrisso Treatment
By Andrew Ciupek, Ph.D., Manager, Clinical Research, GO2 for Lung Cancer Approximately 30% of Non-Small Cell Lung Cancers (NSCLC) have an EGFR mutation. For these patients EGFR targeted therapy has greatly increased survival. Tagrisso (osimertinib) has become the drug of choice for EGFR+ (positive) NSCLC treatment after the FLAURA [...]
Update for Week of Jan 11 to the Joint Statement on COVID-19 From Lung Cancer Advocacy Groups
Happy New Year! We recognize that this year is starting on a somber note. We are still witnessing the unfolding holiday surge. As of January 7, we stand at 21.3 million COVID-19 cases in the US, with 361,312 deaths. Some health systems are on the brink of collapse. In [...]
Patients First
Australian Lisa Briggs is proof that living on a different continent is no impediment to connecting with GO2 for Lung Cancer. In 2014, after months of misdiagnosis, she learned she had stage IV lung cancer, ALK-positive. The 32-year-old healthcare professional, wife, and mother of a toddler and a newborn [...]
Hope Rising
As we begin our new year, there has been a word floating in the air and in our conversations that has been missing from our thoughts for a long time: Hope. We have hope that we are finally seeing a way past an incredibly stressful and difficult year of [...]
Caregiver Award Winner on Learning to Appreciate Each Day
For Wayne Yeung, being a caregiver means learning to live with uncertainty. His wife, Rebecca, was diagnosed with lung cancer in 2017. He’s been on the cancer journey with her from Day 1. Yeung is a winner of the 2020 GO2 for Lung Cancer Wind Beneath My Wings Caregiver [...]
New Events Coming in 2021
As we enter the start of a fresh, new year, here’s a sneak peek of the exciting new opportunities and familiar favorites we will be bringing you in 2021 with GO2 for Lung Cancer Events: Join us as we kick off Spring with a NEW [...]
New Immunotherapy Research is Published – Powered by You. Thank you!
This past week, the first scientific publication from the Lung Cancer Registry (www.lungcancerregistry.org) was published in the journal Cancers in a special edition on symptoms and side effects in cancer survivors. The study was led by Heather Jim, PhD of Moffitt Cancer Center and Adam Dicker, MD, PhD of [...]
January is National Radon Action Month
By Gloria Linnertz, Founder/Director Citizens for Radioactive Radon Reduction Being diagnosed with lung cancer is a drastic way to discover the danger of radioactive radon gas exposure. Most people don’t know that radon gas is a leading cause of lung cancer and that it is present in elevated levels [...]
Another Step for Early Detection
We are proud to support S. 5051, the Multi-Cancer Early Detection Screening Coverage Act and applaud the leadership U.S. Senators Mike Crapo (R-Idaho), Michael Bennet (D-Colorado), Tim Scott (R-South Carolina) and Ben Cardin (D-Maryland). This step follows an early December introduction of H.R. 8845 in the House of Representatives. [...]
Together, We Did It—Restoration of $20M!
This week, Congress completed negotiations on the federal budget and very early today passed the Omnibus Appropriations bills as well as a $900 billion pandemic relief bill. The bill on its way to the President’s desk includes the DEPARTMENT OF DEFENSE APPROPRIATIONS BILL for Fiscal Year 2021, retaining the [...]
First Targeted Adjuvant Therapy for EGFR Non Small Cell Lung Cancer
On December 18th, 2020, the Food and Drug Administration (FDA) approved Tagrisso (osimertinib) for the adjuvant treatment of stage IB-IIIA non-small cell lung cancer (NSCLC) that has been surgically removed, when the cancer has a change in EGFR. The FDA based their approval on the results of the clinical [...]
Survivor Spotlight: Julian F.
My name is Julian Fletcher and I live in Bowie, MD. I was diagnosed on March 12, 2019 with Stage 4 non-small cell lung cancer with a Her2 mutation. I’ve been inspired throughout my journey by the gratitude I have for the people in my life. The support system [...]
Update for Week of Dec 14 to the Joint Statement on COVID-19 From Lung Cancer Advocacy Groups
The first case of COVID-19 in the USA was reported on 1/20/2020—over 10 months ago. Since then, the country has reported 15,718,811cases and 294,535 deaths as of December 12 (per the Centers Disease Control and Prevention). With 80% of US counties reporting more travel than last year over Thanksgiving weekend [...]
Removing Barriers to Screening: A Policy Update
We are proud to support H.R. 8845, the Multi-Cancer Early Detection Screening Coverage Act and applaud the leadership of U.S. Reps. Terri Sewell (AL-07), Jodey Arrington (TX-19), Raul Ruiz (CA-36). GO2 for Lung Cancer’s overarching mission is to transform survivorship for those impacted by the disease. A cornerstone to [...]
A Silver Lining: 2020 Treatment Advancements
In a year full of challenges for everyone, there was a silver lining for lung cancer patients in the form of the abundance of new approvals by the Food and Drug Administration (FDA). In 2020 there were approvals for both new immunotherapies as well as targeted therapies that provide [...]
Lung Cancer and Precision Medicine – Past, Present and the Future of Health Care
Hippocrates made a keen observation more than 2,400 years ago, “It is more important to know what kind of patient has the disease than what kind of disease the patient has.” This statement could not ring truer then as it does now. It is the driving force behind [...]
15th Annual Virtual Gala: A Smashing Success
Thank you to everyone who sponsored, donated and participated in Simply the Best XV Virtual Gala on November 14, 2020. Your generous donations helped raise over $650,000 for the patient programs and lung cancer research for GO2 for Lung Cancer. The support [...]
Veteran Spotlight: Patrick
My name is Patrick and I am a US Navy Aircrew Veteran from 1959-1965, a member of Air Antisubmarine Warfare Squadron VS32, flying in Grumman S2F’s attached to the aircraft carrier USS Lake Champlain CVS 39. I never imagined that my journey in my golden years would be [...]
Update for Week of Nov 23 to the Joint Statement on COVID-19 From Lung Cancer Advocacy Groups
We are at a critical moment in the ongoing COVID-19 pandemic. New cases are rapidly escalating throughout the country, and we are positioned to see explosive growth as people travel and gather to celebrate the Thanksgiving holiday with loved ones. While our understanding of how to treat COVID-19 has [...]
Veteran Spotlight: Joseph
My name is Joseph and I am a Navy Veteran. My beautiful wife Kimberly had back pain that increased over a year which I would help her massage to relieve nightly. We mistakenly overlooked this as a minor issue until it became unbearable. At that point, we went to [...]
About That Other “C” Word (COVID-19)
While we entered the year 2020 celebrating the largest one-year drop in cancer mortality, it wasn’t long before a new “C” entered the national conscience: COVID-19. For the lung cancer community in particular, COVID-19 presents a unique threat. Several studies have reported that patients with lung cancer who develop [...]
Pass the Resolution Recognizing November as Lung Cancer Awareness Month!
Today, a Senate Resolution was introduced as the companion to the House Resolution (H. Res.1192), supporting the goals of Lung Cancer Awareness Month (LCAM) and the early detection of lung cancer. TAKE ACTION! The Resolution will support LCAM goals to: Designate November 2020 as the National Lung [...]
GO2 for Lung Cancer Recognizes the Lung Cancer Community at Simply the Best XV Virtual Gala
SAN CARLOS, Calif. and WASHINGTON, D.C., November 14, 2020 – GO2 for Lung Cancer (GO2 for Lung Cancer) will honor leaders in lung cancer research and treatment, as well as dedicated lung cancer caregivers, at its Simply the Best XV Virtual Gala tonight. "The GO2 for Lung Cancer annual [...]
Delivering Excellence in Care Closer to Home
As we enter Lung Cancer Awareness Month, our mantra of “Lung Cancer: It’s Personal” could not have been on display better than during our recent 2020 Centers of Excellence (COE) Summit. This event brought together more than 300 healthcare providers, industry partners and patients to discuss successes, innovations and [...]
A Veteran, Vietnam, and Lung Cancer
My name is Fred Rothaermel and I live in Charleston, South Carolina. In 1966 I enlisted in the United States Marine Corps, and soon enough found myself in Vietnam, where I spent three tours where I was exposed to “harmless” Agent Orange along with many of my Brothers. I [...]
Update for Week of November 9, 2020 to the Joint Statement on COVID-19 From Lung Cancer Advocacy Groups
The first case of COVID-19 in the USA was reported on 1/20/2020—over 9 months ago. Since then, the country has reported 9,860,558 cases and 237,113 deaths (per Johns Hopkins). As the weather becomes cooler and we spend more time indoors, the number of cases is rapidly accelerating in almost [...]
There’s Nothing Typical About These Athletes
With most spring and fall endurance races cancelled this year, many races offered a virtual option. At GO2 for Lung Cancer, we had over a dozen endurance athletes already committed, training, and fundraising for “bucket list” fall races including a half Ironman, the Marine Corps Marathon, and New York [...]
Lung Cancer Awareness Month – It’s Personal
November is Lung Cancer Awareness Month (#lcam), a time for us to pull out the bullhorn and amplify our collective voices to speak up and speak out about the importance of screening, comprehensive biomarker testing, research, funding, community engagement, and an end to the stigma surrounding lung cancer.Awareness months [...]
Bench to Bedside and Back Again
Today’s blog presents Christine Lovly, MD, PhD, Associate Professor of Medicine, Ingram Associate Professor of Cancer Research, and Co-leader of the Translational Research and Interventional Oncology Research Program at Vanderbilt University Medical Center. Dr. Lovly was recently named Chair of our Scientific Leadership Board. She is a highly recognized oncologist and [...]
A Message of Patient Empowerment
An informed patient is an empowered and hopeful patient. Our co-founder and board chair Bonnie J. Addario has led efforts to transfer knowledge and inspire optimism through articles, interviews, and regular episodes of the Lung Cancer Living Room. We’ve curated these resources to bring you illuminating reading and viewing [...]
3 Facts About Clinical Trials Every Patient Should Know
The World Health Organization defines a clinical trial as “a type of research that studies new tests and treatments and evaluates their effects on human health outcomes.” Put simply, it’s a research study involving people, often used to evaluate the safety and effectiveness of new drugs and other therapies. [...]
One Woman’s Voice Makes a Difference
Hildy Grossman’s life has been about caring for people and bringing joy into their lives. She has two professions: clinical psychologist and professional singer. As she shares, “As a psychologist, I have the honor of being invited into people’s lives and connecting [...]
GO2 for Lung Cancer “Simply the Best” XV Virtual Gala Honors Those Working on the Front Lines to Fight Lung Cancer
SAN CARLOS, Calif. and WASHINGTON, Oct. 22, 2020 /PRNewswire/ -- GO2 for Lung Cancer announced it will celebrate Genentech with the "Simply the Best Award" at its XV Virtual Annual Simply the Best Gala on November 14, 2020 at 6:30 pm PT. Genentech will be honored for its continued dedication to research and development of new lung cancer treatments. [...]
Christine M. Lovly, MD, PhD, to Chair Scientific Leadership Board
SAN CARLOS, Calif. and WASHINGTON, Oct. 21, 2020 /PRNewswire/ -- GO2 for Lung Cancer is pleased to announce that Christine Lovly, MD, PhD, has been named as the incoming chair of GO2 Foundation's Scientific Leadership Board (SLB). Dr. Lovly, an associate professor of Medicine, Ingram Associate Professor of Cancer Research, and co-leader of the Translational [...]
October 19 Update on Coronavirus and Lung Cancer
The daily news reports are a stark reminder that the COVID-19 pandemic is far from over. Consistent with experts’ fears for the fall, new cases are on the rise across the US and in Europe. Caught in the grips of this unprecedented public health crisis for almost all [...]
The Voice of Hope
By: Laurie Fenton Ambrose, Co-Founder, President and CEO, GO2 for Lung Cancer Our Co-Founder and Board Chair Bonnie J. Addario is the recipient of the 2020 Lifetime Achievement Award from CURE Media Group. The award is part of the inaugural The 2020 Lung Cancer Heroes™ virtual celebration being held [...]
30 Day Hope and Wellbeing Challenge
We can all agree that 2020 has been an incredibly challenging year. Recognizing the stress that many are feeling, we want to encourage you to take care of yourself during Lung Cancer Awareness Month (LCAM). This year’s theme is “It’s Personal” and personal self-care has never been a more [...]
Comprehensive New Initiative to Study COVID-19 and Lung Cancer
We are pleased to announce that GO2 for Lung Cancer is part of a recently-awarded five-year grant from the National Cancer Institute as part of its new SeroNet initiative. This project seeks to uncover whether people with lung cancer, who are uniquely at risk for worse outcomes from COVID-19, [...]
Breathing and Believing in Hollywood, FL
When Bonnie Bishop-Ulrich found out she had stage 3 non-small cell lung cancer, she literally fell to her knees. She couldn’t bear the thought of missing her grandchildren grow up and took a leap of faith to join GO2 for Lung Cancer’s virtual Hollywood, Florida [...]
Updates from GO2 for Lung Cancer’s Centers of Excellence
GO2 for Lung Cancer Welcomes Two New Centers of Excellence Last month GO2 for Lung Cancer welcomed two new Centers of Excellence (COE) in Lung Cancer Screening: Robert J. Dole VA Medical Center in Wichita, Kansas and Hunter Holmes McGuire VA Medical Center in Richmond, VA. We are thrilled [...]
Bring Extra Meaning to the Holidays
This year’s coming holiday season may have you focused differently than in years past. At a time when it is easy to get overwhelmed, it is important to focus on the small, tangible things that bring you joy. Despite all the chaos and uncertainty [...]
How Screening Centers Are Bringing Patients Back
Catching Lung Cancer Early Remains Critical Tool in Fighting the Disease When the Coronavirus shutdowns hit, a lot of primary and preventive care shut down with the pandemic. Slowly, as clinicians switched to telehealth appointments and facilities set up protocols to protect non-COVID-19 patients, patients started returning for treatment [...]
Practicing Medicine for the Patient
Narjust Duma, MD, Principle Investigator of the SHAWL Study Today’s blog presents Narjust Duma, MD, Assistant Professor, Division of Hematology/Oncology, Medical Oncology, and Palliative Care, University of Wisconsin School of Medicine and Public Health, University of Wisconsin Carbone Cancer Center, Madison, Wisconsin. Dr. Duma (pictured left) is [...]
The Complete Story—GO2 for Lung Cancer and Biomarker Testing
Comprehensive biomarker testing identifies what is unique about a lung cancer patient’s cancer, ensuring they receive appropriate treatment. Increasing the use of comprehensive biomarker testing is one of GO2 for Lung Cancer’s key goals. To reach it, we’re using a multipronged approach that includes patient education, assessing data on [...]
Update for Week of October 5, 2020 to the Joint Statement on COVID-19 From Lung Cancer Advocacy Groups
As of October 3, 2020, the US has had close to 7.2 million cases of COVID-19, with over 200,000 deaths. Daily reported cases of COVID-19 have been on the rise. This is not surprising due to social distancing fatigue and mask fatigue. As the weather becomes cooler and we [...]
Lung Cancer Awareness in the Time of COVID-19
This is an unusual year for us all, but healthcare facilities are still focused on lung cancer education and awareness. To help them in this effort, we offer our Shine a Light on Lung Cancer program that provides hospitals with guidance and supportive educational materials for use during Lung [...]
Angela Criswell Wins the the 2020 Timothy W. Mullett, MD, Lung Cancer Prevention Award
Angela Criswell, MA, Associate Director of Quality Screening & Program Initiatives at GO2 for Lung Cancer has been selected to receive the 2020 Timothy W. Mullett, MD, Lung Cancer Prevention Award. This award is given in recognition of outstanding leadership, devotion, and passion toward preventing lung cancer through education, advocacy, [...]
5 Tips to Help Your Loved One with Lung Cancer
When a loved one finds out they have lung cancer it can be hard to know how best to help them, what to say or even how they are feeling. Here are five helpful tips for being the best caregiver to your loved one. Take care of yourself. There is [...]
Bonnie J. Addario Announced as Winner of Lifetime Achievement Award from CURE Media
Congratulations to our own Bonnie J. Addario for receiving the Lifetime Achievement Award from Cure Magazine. We are so proud! Bonnie’s awe-inspiring message of empowerment has reached millions. CRANBURY, N.J.--(BUSINESS WIRE)--CURE Media Group, the industry-leading multimedia platform devoted to cancer updates and research that reaches more than 1 million [...]
Update for Week of September 21, 2020 to the Joint Statement on COVID-19 From Lung Cancer Advocacy Groups
As of September 18, 2020, the US has had 6.7 million cases of COVID-19, with just over 198,000 deaths. The Midwest is leading new cases, with 8 cities in Wisconsin appearing on The New York Times list of the 20 metro areas with fastest-growing cases. With the run-up to [...]
Are You Biomarker Savvy?
Every person’s cancer is different. Between all of the lung cancer treatment options available, how can you and your treatment team identify the best path for your individual case? Enter: comprehensive biomarker testing. Comprehensive biomarker testing (also called molecular testing) looks for biological changes, like EGFR or ALK, that [...]
FDA Approves Gavreto (pralsetinib) for the Treatment of Advanced NSCLC with Tumors Having Changes in RET
On September 4th, 2020, the Food and Drug Administration (FDA) approved Gavreto (pralsetinib) for the treatment of advanced non-small cell lung cancer (NSCLC) with tumors having changes in RET. The FDA based their approval on the results of the clinical trial ARROW which showed that patients receiving Gavreto (pralsetinib) [...]
September 7 Update on Coronavirus and Lung Cancer
We hope that all of you had a peaceful Labor Day holiday. This week marks the six-month anniversary of when the World Health Organization declared COVID-19 a global pandemic (March 11). As of September 7, 2020, cases in the US have surpassed the 6 million mark, with over 186,000 [...]
Elite Swimmers Honor One of Their Own
Through our community fundraising program, dedicated and caring people can turn their personal passion into a fundraiser. For the past six years, San Francisco area resident Glenda Carroll has been event director for Tamalpais Aquatic Masters (TAM) for an annual elite swimming fundraiser to honor fellow swimmer [...]
New FDA Approval for FoundationOne Liquid CDx Biomarker Testing
On August 26th, 2020, the Food and Drug Administration (FDA) approved the FoundationOne Liquid CDx for comprehensive liquid biopsy biomarker testing for patients with any solid tumor. The FDA based their approval on analysis from multiple clinical validation studies which showed that FoundationOne Liquid CDx is very sensitive and [...]
Reclaim 2020 and Find Your Event
This year has been many things, but missing for many of us has been the element of choice. In putting together our event offerings for this fall, we wanted to give you the opportunity to find what works best for you and to customize your experience with the lung [...]
Lung Cancer Screening Eligibility
The life-saving benefit of finding and treating lung cancer early is undeniable. That is why GO2 for Lung Cancer’s community advocates for screening and the expansion of this benefit to more people at risk. It is also why we work to instill responsible screening and care by growing a [...]
A Case Study in Excellence: Southern Ohio Medical Center
Portsmouth, OH, and the surrounding region has a disproportionately high number of people diagnosed with lung cancer. The screening rate at Southern Ohio Medical Center (SOMC) is 12.28 cases per 1,000. The national average: 2.61. It’s a community in stark need of great medical care. Fortunately, the people there [...]
Leave No Gene Behind
The lung cancer world now has therapies tied to individual biomarkers, making comprehensive biomarker testing an imperative at diagnosis. On August 18, our monthly Lung Cancer Living Room featured Dr. Benjamin Levy from Johns Hopkins University/Sidney Kimmel Comprehensive Cancer Center for a discussion on “New Biomarkers/New Therapies and the [...]
August 24 Update on Coronavirus and Lung Cancer
It has been more than 6 months since the first cases of COVID-19 hit the United States. We issued our first update on March 3, a week before the World Health Organization declared a global pandemic on March 11. As of August 24, 2020, cases in the United States [...]
Recognizing Excellence
GO2 for Lung Cancer is proud to recognize the Central Virginia VA Health Care System (CVHCS), Hunter Holmes McGuire VAMC as currently the only VA medical facility in the country to be designated as a Screening Center of Excellence within our Centers of Excellence: Screening and Care Continuum national network. [...]
Keith Perry: Endurance Athlete, Husband, Dad, Friend, and Stage 2 Lung Cancer Patient
Like many seasoned athletes, the last thing on Keith Perry’s radar was developing lung cancer. But less than two years ago, he received that diagnosis and believed his racing days were over. But despite a stage 2 diagnosis and a full left pneumonectomy, followed by chemotherapy [...]
This Physician-Scientist Wants to Personalize Radiation Treatment
Dr. Moding, recipient of the 2020 Young Investigator Award Which patients respond to radiation treatment and why? That’s the question that Everett Moding, MD, PhD, is trying to understand. Dr. Moding, recipient of the 2020 GO2 for Lung Cancer / ASCO Foundation Conquer Cancer Young Investigator Award, is [...]
Making Memories and Honoring Your Loved One
Your special day can help fund GO2 for Lung Cancer programs, while honoring someone special. Alyssa Vasquez Nichols shares why and how she chose to incorporate giving into her own wedding this past fall. "My mom was diagnosed with Stage IV NSCLC in 2009. When [...]
Lung Cancer Staging 101
Why read up on lung cancer staging? To start, staging is an incredibly important step in your diagnosis. It’s impossible to know the best treatment option for you or your loved one without knowing the stage of the cancer, as treatments differ from stage to stage due to FDA [...]
FDA Approval: Access to New Comprehensive Liquid Biopsy Biomarker Testing
On August 7th, 2020, the Food and Drug Administration (FDA) approved the Guardant 360 CDx for comprehensive biomarker testing for patients with any solid tumor. The FDA based their approval on data from the clinical trials FLAURA and AURA3 as clinical validation that patients receiving liquid biopsy biomarker testing [...]
August 10 Update on Coronavirus and Lung Cancer
As of August 9, 2020, we are approaching 20 million cases of COVID-19 worldwide, with almost 5 million cases and 160,000 deaths in the US alone. In this week’s update, we want to shift our attention to another looming healthcare crisis resulting from the pandemic, namely a significant decline [...]
Survivor Spotlight: I’m Still Worth It
My name is Trish Hom. Patricia Hom. Dr. Patricia Hom. I am an MD/MPH. I had been coughing for six months during my OBGYN residency. Two weeks before I graduated residency my children’s father committed suicide. I was very sick at that time, and it was the loneliest plane [...]
Detection and Treatment: Four Easy Steps
We kicked off August raising awareness during World Lung Cancer Day. Now it’s time to get more personal and focus on the important steps to take to effectively detect and treat lung cancer. Here are four easy steps any survivor, advocate, or caregiver can take during the month of [...]
Leading the Charge in Houston
As event day approaches for the 7th Annual GO2 for Lung Cancer Houston event, we took some time to catch up with Janie Aranda and Kristy Rogers, the two founding members of the Houston Walk/Run. What is your favorite aspect of serving on [...]
Your Voices Were Heard! Congressional Representatives Restoring $20M!
We are excited to share that the House of Representatives has passed the DEPARTMENT OF DEFENSE APPROPRIATIONS BILL that retains the full allocation of $20 million for the Lung Cancer Research Program (LCRP). Also included in the bill, is “Report Language” to authorize the CDC to promote lung cancer [...]
Women and Lung Cancer and Congress
How and why lung cancer affects women differently than men – and what we need to do to better address this women’s health imperative – has been a central focus of ours for years. Amid our efforts to keep our community apprised of COVID-19 safety measures, new lung cancer treatments, [...]
My Story Can Inspire
Sydney Yolande Barned, MD, is a practicing physician at Anne Arundel Medical Center in Annapolis, Maryland. She is also a lung cancer patient and an advocate. We asked her to share some insights from her unique perspective as a patient and a physician. Q: Dr. Barned, please tell us [...]
Survivor Spotlight: Roy W.
My name is Roy Williams III and I live in the St. Louis, Mo area. My wife Mary and I have six children in our blended family and four grandchildren. In 2013, when I was 64, I developed an upper respiratory infection. I went to my primary care physician [...]
July 27 Update on Coronavirus and Lung Cancer
These updates began on March 3, 2020--a week before the World Health Organization declared COVID-19 a pandemic--when concerns arose in the lung cancer community regarding news out of China about a novel respiratory virus especially deadly to patients with lung cancer. Dr. Upal Basu Roy (who holds a [...]
Veteran Spotlight: John
My name is John Casterline and I am a US Navy Veteran. I retired from the Navy in 1986 after 21 years then retired a second time from 7-Eleven in 2006 after 21 years. Just seven days later I was diagnosed with cancer. One week later I was told [...]
Turn Your Passion Into Progress
What does a birthday, handmade jewelry, a wedding, an outdoor yoga class and a cancerversary all have in common? They can all be opportunities to fundraise for more breakthroughs in lung cancer treatment. Adding a fundraising component to what you are already doing is easier than you think! Every [...]
GO2 for Lung Cancer to Honor Charles Swanton, M.D., Ph.D., FRCP, FMedSci, FRS with the 2020 Bonnie J. Addario Lectureship Award
GO2 for Lung Cancer recognizes Professor Swanton for genetic research transforming lung cancer treatment San Carlos, CA and Washington, D.C., July 23, 2020 – GO2 for Lung Cancer (GO2 for Lung Cancer) will present Charles Swanton, M.D., Ph.D., FRCP, FMedSci, FRS senior group leader at the Francis Crick Institute [...]
Using Donations Wisely to Accomplish Our Life-Saving Mission
In recognition of its strong financial health and commitment to accountability, GO2 for Lung Cancer received the highest non-profit rating from the nation’s largest evaluator of charities, Charity Navigator. This is the 7th consecutive time that GO2 for Lung Cancer has earned this top distinction of 4-Stars. Since 2002, [...]
Celebrating Parents, Honoring Survivors
Being a parent comes with a unique set of challenges—but being a parent with lung cancer adds another layer of physical and emotional exhaustion. In celebration of Parents Day 2020, we are highlighting the unsung heroes in our community who have many accolades, including both parent AND lung cancer [...]
Ambassadors with a Mission
By Nicole Phipps, Director, Community Engagement When GO2 for Lung Cancer was formed in April 2019, we brought together trusted and long-tenured advocates, volunteers, fundraisers, and supporters to form a National Ambassador Council (NAC) to amplify our efforts to reach the broadest community. This group of lung cancer patients, [...]
Survivor Spotlight: Jeff M.
Written by Jeff's wife, Rhonda: "Jeff was a firefighter/paramedic and a farmer and had no known risk factors for developing lung cancer. In the fall of 2015, my husband Jeff was diagnosed with stage 4 ALK-positive lung cancer that had already spread to his bones and brain at the [...]
Advocacy Impact Made Tangible
The impact of raising our voices together at the June Virtual Lung Cancer Voices Summit has been huge! So we are sharing more tangible outcomes of the Summit with you today. Recognition from the Congressional Lung Cancer Caucus We couldn’t hear from our Congressional Lung Cancer Caucus Chairs in [...]
My Story: Ride Hard, Breathe Easy
We all have different interests and gifts to bring to the lung cancer movement. For so many, we want to do SOMETHING, but not sure what or how. John Matthews was in this position, and has since found his way to raise an incredible amount of money, while keeping [...]
Getting Close to Restoring $20 Million
Getting Close to Restoring $20 M! We have cleared the second and are close to the final hurdle in the House of Representatives to restoring and securing $20 million in federal funding for lung cancer research! What Happened! Today, the House of Representatives Appropriations Full Committee, Chaired by Representative [...]
Moving Forward
The Monthly Measure will be delivered in the first week of the month. Each issue will contain articles highlighting our work and our people. Expect to see: a newsworthy event, a unique perspective on an ongoing program, a profile of one of the people making a difference, and a link to [...]
New Lung Cancer Registry Study on Women and Sexual Health
No one asks women with lung cancer about their sex lives—until now. A new study from the Lung Cancer Registry explores sexual dysfunction in women with lung cancer in hopes of better understanding the problem and finding solutions. The study, Sexual Health Assessment in Women with Lung Cancer (SHAWL), [...]
A Conversation with Our Chief Scientific Officer
On why she studies lung cancer & what she values in talking to patients. MM [Monthly Measure]: What brought you into the cancer space? Two things. I've been a math and science person since I was a little kid. As I got older I became interested in the questions [...]
Serving Our Nation’s Veterans
Men and women who served in the military, especially those who served in combat, are at higher risk of lung cancer than civilians. For well over 15 years, GO2 for Lung Cancer has made screening and care for our military men and women a core priority. We are committed [...]
GO2 for Lung Cancer’s Virtual 5K Was A Success in More Ways Than One
It is often said that no one would choose to be a member of the lung cancer community, but a common silver lining is that some of the strongest relationships are forged among patients and caregivers. There is an instant kinship from shared experience that is priceless. We know [...]
July 13 Update on Coronavirus and Lung Cancer
As of July 1, 2020, more than 10 million people worldwide have been infected with SARS-CoV-2, the virus that causes COVID-19. In the United States alone, more than 3 million people have tested positive, as of July 10, 2020. Our knowledge about how the virus affects our immune systems [...]
Our Voices Were Heard – $20 Million More!
Our voices were heard! We have cleared the first important hurdle to restoring and securing $20 million in federal funding for lung cancer research. Today, the House of Representatives Appropriations Subcommittee on Defense passed its fiscal year 2021 Bill that funds the Lung Cancer Research Program (LCRP), within the [...]
Veteran Spotlight: Julio
My name is Julio Sanchez, a "Soldier for Life" after proudly serving 28 years in the US Army as a sapper or combat engineer. I live in the Fort Benning (“The Home of the Infantry”) area of Columbus, Georgia. I retired in March 2007. I am married, have two [...]
New Draft Recommendation Expands Eligibility for Those at High Risk for Lung Cancer
A step forward in preventative screening for lung cancer. The U.S. Preventive Services Task Force (USPSTF) issued its 2020 draft recommendation on annual lung cancer screening using low-dose computed tomography (LDCT) today. This draft recommendation will replace the 2014 recommendation that established LDCT lung cancer screening as a recommended preventive [...]
Fundraiser Spotlight: Following in Superman’s Path
Meet Carol Lendle, captain of Eau Claire Half Marathon Team Breathe: The inspiration for becoming a lung cancer advocate is my late brother Don Stranathan. He was diagnosed in June of 2009 with stage 4 NSCLC. He lived almost 11 years after that diagnosis and became a selfless advocate [...]
June 29 Update on Coronavirus and Lung Cancer
As of June 28, 2020, the United States has reported more than 2.5 million cases of COVID-19 and 125,484 deaths. We are now seeing a rapid escalation in cases in states across the US. Some would argue that these increases simply reflect more testing but that only tells part [...]
You Made Your Voices Heard!
Our community’s advocates made this year’s Virtual Lung Cancer Voices Summit a huge success. Social distancing required us to quickly transition to a virtual advocacy event for 2020, but we rallied and the lung cancer community roared! Let’s look at some of the numbers: Registered Advocates: 230 including 161 [...]
Lung Cancer Registry Launches Landmark Survey on Women’s Sexual Health
WASHINGTON, June 23, 2020 /PRNewswire/ -- The Lung Cancer Registry has launched a landmark new survey on the impact of lung treatments on women's sexual health. The aim is to explore the magnitude of the problem and give researchers and clinicians new insights to improve the quality of life for women lung cancer [...]
Veteran Spotlight: Ken
My name is Ken Wheatley and I live in San Diego, California. I’m a veteran and served as a Vietnam-era non-commissioned officer (NCO) in the Air Force. I worked inertial guidance, forward-looking radar, and bombing computer systems on F-4 jets. Sheila and I dated for several years and got [...]
Veteran Spotlight: Curt
I joined the Air Force just three days after graduating from high school in 1967. I spent four years in the Air Force, volunteered for a special unit called Safe Side, went to Vietnam, then was stationed in Florida and Louisiana before being discharged in 1971. Later, because of [...]
Veteran Spotlight: Larry
My Story My name is Larry Gershon. I am 72 years old, live in Palo Alto, California, and have been married to my wife for 46 years. In addition to being a husband, father of two, and grandfather, I am also a veteran. I retired from the military in [...]
Veteran Spotlight: Roger
As the husband of a lung cancer survivor, I know that so much more needs to be done to accurately diagnose and treat this terrible disease. It took nine months before there was a definitive diagnosis of my wife’s first lung cancer tumor; six months later she faced a [...]
FDA approves pembrolizumab for adults and children with TMB-H solid tumors
On June 16th, 2020, the Food and Drug Administration (FDA) approved Keytruda (pembrolizumab) for treatment of all advanced, metastatic cancers with a tumor mutational burden-high (TMB-H) designation. The FDA based their approval on the results of the clinical trial KEYNOTE-158 which showed that a high percentage of patients receiving [...]
Worth The Fight: Taking On Lung Cancer
Jodi Parker, a former oncology nurse who was originally diagnosed with Stage 4 non-small cell lung cancer in 2013, sat down with her son Joey, one of her primary caregivers, to discuss what motivated her to take an active role in her cancer care. Parker has teamed up with [...]
VA and GO2 for Lung Cancer Partner to Improve Outcomes for Veterans at Risk of Lung Cancer
A historic milestone was reached today with the exciting announcement that the Department of Veterans Affairs (VA) and GO2 for Lung Cancer (GO2 for Lung Cancer) have established a formal partnership to advance lifesaving screening and care for our military men and women at greater risk for lung cancer. [...]
FDA approves Zepzelca (lurbinectedin) for treatment of advanced SCLC
On June 15, 2020, the Food and Drug Administration (FDA) approved Zepzelca (lurbinectedin) for treatment of advanced small cell lung cancer (SCLC) whose cancer has progressed after platinum chemotherapy. The FDA based their approval on the results of a clinical trial which showed that a high percentage of patients [...]
Improving Lung Cancer Screening For Our Veterans: An Update
By: Angela Criswell, Associate Director, Quality Screening and Program Initiatives, and Anita McGlothlin, Director, Economics & Health Policy Lung cancer among veterans is a serious issue because they face higher incidence rates of the disease and a younger median age at diagnosis compared with the general US population. The [...]
June 15 Update on Coronavirus and Lung Cancer
As of June 12, 2020, the United States has reported more than 2 million cases of COVID-19 and 113,914 have died from this disease. States are in different phases of reopening and shelter-in-place restrictions and lockdown have been eased in almost every state in the USA. With restrictions being [...]
4 Ways to Make Your Voice Heard for Lung Cancer Research
Help shape the future for millions of Americans at risk or living with lung cancer. Make your voice heard demanding vital federal funding for lung cancer research. This year we’re requesting $20 million to increase the Lung Cancer Research Program—the largest lung cancer program outside of the National Cancer [...]
Fundraiser Spotlight: Terri Ann DiJulio
When you meet Terri Ann DiJulio, words like vivacious and fit come to mind. You wouldn’t know the words two-time lung cancer and stroke survivor also fit. We recently connected to talk about her advocacy and plans to raise $60,000 for GO2 for Lung Cancer this year. Q: What [...]
What You Need to Know: ASCO 2020
By Rashmi Acharya, MS; Andrew Ciupek, PhD; Jennifer C. King, PhD; Amy C. Moore, PhD; Daniel Saez, MS; Jacinta Wiens, PhD This past weekend was the ASCO Annual Meeting with presentations of some of the most important lung cancer data of the year. GO2 for Lung Cancer’s Science & [...]
We Stand Together
At GO2 for Lung Cancer we believe when social injustice rules whether through stigma, unequal access to healthcare, or violent and discriminatory actions, no one wins. We must stand together now, change this wrong, and unite as a nation that is caring, respectful and supporting of all people. We [...]
FDA Approves ramucirumab Plus erlotinib for First-Line Metastatic NSCLC
On May 29, 2020, the Food and Drug Administration (FDA) approved the combination of Cyramza (ramucirumab) and Tarceva (erlotinib) for the first line treatment of advanced non-small cell lung cancer (NSCLC) with tumors having changes in EGFR called exon 19 deletions or exon 21 L858R mutations. The FDA based [...]
June 1 Update on Coronavirus and Lung Cancer
This past week marked a grim milestone in the United States, as we officially surpassed 100,000 deaths from COVID-19. Our groups continue to recommend that the lung cancer community adhere to best practices to limit exposure, including wearing masks/face coverings when out in public, frequent handwashing, ongoing social distancing, [...]
You’re Invited to the 2020 Virtual Lung Cancer Voices Summit—Join Us
Now, more than ever, we must raise our collective voices to protect vital federal research funding for lung cancer. Help us reach lawmakers on Capitol Hill to urge them to fully fund the Lung Cancer Research Program. We’re holding a Virtual Lung Cancer Voices Summit this year—and you’re invited [...]
FDA Approves nivolumab Plus ipilimumab and Chemotherapy for First-Line Treatment of Metastatic NSCLC
On May 26, 2020, the Food and Drug Administration (FDA) approved the combination of Opdivo (nivolumab), Yervoy (ipilimumab), and two cycles of platinum-doublet chemotherapy for the first line treatment of advanced non-small cell lung cancer (NSCLC) with tumors having no changes in EGFR or ALK. The FDA based their [...]
FDA Approves brigatinib for ALK-positive Metastatic NSCLC
On May 22, 2020, the Food and Drug Administration (FDA) approved Alunbrig (brigatinib) for the first line treatment of advanced non-small cell lung cancer (NSCLC) with tumors having changes in ALK. The FDA based their approval on the results of the clinical trial ALTA 1L which showed that patients [...]
Science Doesn’t Stop!
By Jennifer C. King, PhD, Chief Scientific Officer I’m an optimist at heart -- to the point where my tween daughter just rolls her eyes at me whenever I tell her to have a more positive attitude. Consequently, while it feels like all the news has been negative and [...]
Why I Run
Everyone has a story to share. Your voice. Your experiences. Your memories. They are uniquely yours and yet they have the power to build bridges, forge friendships and show others they are not alone.For this year’s Summer Jam Virtual 5K Your Way, we are encouraging everyone to share their story [...]
FDA Approves Tecentriq for First-Line Treatment of Metastatic NSCLC with High PD-L1 Expression
On May 18th, 2020, the Food and Drug Administration (FDA) approved Tecentriq (atezolizumab) for the first line treatment of advanced non-small cell lung cancer (NSCLC) with tumors having high expressions of PD-L1 (>50%) and no changes in EGFR or ALK. The FDA based their approval on the results of [...]
May 18 Update on Coronavirus and Lung Cancer
As different states are relaxing shelter-at-home orders and businesses are planning to re-open, it is important to understand the true extent of COVID-19 infections: both active infections (patients who are currently infected) and past infections (patients who were infected in the past and have now recovered). Currently, active infections [...]
First FDA Approval of Combined Immunotherapy Treatment for NSCLC
On May 15, 2020, the Food and Drug Administration (FDA) approved the combination of Opdivo (nivolumab) and Yervoy (ipilimumab) for treatment of advanced non-small cell lung cancer (NSCLC) with a PD-L1 level equal to or greater than 1% for patients that do not have EGFR or ALK mutations. The [...]
Telehealth Offers a Lifeline Between Patients and their Doctors
In March 1999, physician Jerri Nielsen was stationed at a research facility in Antarctica when she discovered a lump in her breast. For the next seven months, until a plane could safely land in the harsh polar landscape, she treated herself in consultation with an oncologist based in Indiana. The [...]
GO2 for Lung Cancer Names Jennifer C. King, Ph.D. as Chief Scientific Officer
SAN CARLOS, Calif. and WASHINGTON, May 13, 2020 /PRNewswire/ -- GO2 for Lung Cancer (GO2 Foundation) has promoted Jennifer C. King, Ph.D., to the role of chief scientific officer. In her new position, Dr. King will be responsible for leading scientific and research priorities for a global patient advocacy group dedicated to saving, extending, and improving the [...]
FDA Approves Retevmo (selpercatinib) for Treatment of Advanced NSCLC
On May 8, 2020, the Food and Drug Administration (FDA) approved Retevmo (selpercatinib) for treatment of advanced non-small cell lung cancer (NSCLC) with a change in RET under the accelerated approval pathway. The FDA based their approval on the results of the clinical trial LIBRETTO-001 which showed that a [...]
Register Today for the 2020 Virtual Lung Cancer Voices Summit!
REGISTRATION NOW OPEN! This year, we are transforming our annual Advocacy Summit into a free virtual event, June 15-16. Join other lung cancer patients, caregivers, and advocates for an inspiring two-day virtual event to learn about the current state of lung cancer and educate Members of Congress about the [...]
FDA Grants Accelerated Approval to Tabrecta (capmatinib) for Metastatic Non-small Cell Lung Cancer with a Change in MET
On May 6th, 2020, the Food and Drug Administration (FDA) approved Tabrecta (capmatinib) for treatment of advanced non-small cell lung cancer (NSCLC) with a change in MET. The FDA based their approval on the results of the clinical trial GEOMETRY mono-1 which showed that more patients receiving Tabrecta experienced [...]
The New Normal: Lung Cancer and COVID- 19
Dear Lung Cancer Community, Our normal lives have been completely upended by COVID-19. With many activities being deemed “non-essential” it begs the question, what is “essential,” especially when it comes to patient care. Our lung cancer community, which is at increased risk related to Covid-19, has questions and needs [...]
May 4 Update on Coronavirus and Lung Cancer
The authors of this weekly advocacy update are all scientists (nerds) and so before we get to this week’s update, indulge a little humor: “May the Fourth be with you!” Now back to our regularly scheduled programming: As of May 2, 2020, the Centers for Disease Control and Prevention (CDC) [...]
April 27 Update on Coronavirus and Lung Cancer
As of April 24, 2020, the Centers for Disease Control and Prevention (CDC) reports 895,766 cases of COVID-19 and 50,439 COVID-19-associated deaths. As the number of cases continue to rise, the importance of maintaining social distancing and following shelter in place/quarantine orders is central to flattening the COVID-19 curve. [...]
FAQs of Accessing Telehealth
During this unprecedented time, health care is quickly evolving with remote or virtual doctor visits, also known as “telehealth.” Opening the door to telehealth allows patients and providers access to continued care while minimizing the risk of possible exposure to COVID-19 in traditional treatment facilities. We’ve partnered with fellow [...]
April 20 Update on Coronavirus and Lung Cancer
As of April 18, 2020, the Centers for Disease Control and Prevention (CDC) reports 661,712 cases of COVID-19 and 33,049 COVID-19-associated deaths. A recent study conducted by researchers at Stanford University suggests that this number is an underrepresentation of the total number of infected individuals. We must urge caution [...]
Strategies for Coping with Your Emotions in Difficult Times
When we talk about shared experiences, this probably isn’t one any of us would have chosen. But as shelter-at-home orders hit more and more communities, people across the U.S.—and the world—are grappling with our new reality. Maybe this includes you. Resuming a routine can [...]
April 13 Update on Coronavirus and Lung Cancer
As of April 11, 2020, the Centers for Disease Control and Prevention (CDC) reports 492,416 cases of COVID-19 and 18,559 COVID-19-associated deaths. The United States now has the highest number of confirmed COVID-19 cases in the world. These numbers may be an underestimation of the true burden of the [...]
Thank You to the Lung Cancer Community
To our lung cancer community, THANK YOU. Thank you for letting us serve you as the GO2 for Lung Cancer over the last year. Now, more than ever, it is our honor to bring support, information, guidance, and a sense of community to everyone impacted by lung cancer. On April, 8, [...]
COVID-19 and Anti-discrimination – An Update from Leadership
Dear Lung Cancer Community, As COVID-19 continues its spread across our communities, we are all aware that our doctors, nurses, and hospitals are managing an unprecedented crisis that has overwhelmed them and impacted our medical supplies and equipment reserves. We know our care teams are doing everything possible to [...]
April 6 Update on Coronavirus and Lung Cancer
The number of confirmed cases of COVID-19 continues to grow in the US. As of April 5, 2020, there are 312,089 confirmed cases of COVID-19 in the US. These numbers may be an underestimation of the true burden of the disease due to lack of testing and a high [...]
Q&A with Dr. Brendon Stiles: COVID-19 and Surgery
This update has been written in collaboration with Dr. Brendon Stiles. He is Chair of the Board for the Lung Cancer Research Foundation, a thoracic surgeon at New York-Presbyterian Hospital and an Associate Professor of Cardiothoracic Surgery at Weill Cornell Medicine. Q: COVID-19 and surgery: How long can I [...]
Diversion in Isolating Times
By Maureen Rigney, LICSW Director, Support Initiatives GO2 for Lung Cancer The coronavirus and the resulting covid-19 disease have changed everyone’s daily lives. In this blog, Maureen Rigney, our lead social worker and boredom fighter, shares her thoughts on using idle time while at home social distancing. Need a [...]
March 30 Update on Coronavirus and Lung Cancer
As of March 30, 2020, cases of the virus surge in countries around the world. The United States now has the highest number of COVID-19 cases globally. The CDC has issued a travel advisory for the New York tri-state area, which has the highest number of cases in the country. [...]
FDA Approves New First-Line Treatment for Extensive-Stage Small Cell Lung Cancer
On March 27, 2020, the Food and Drug Administration (FDA) approved Imfinzi (durvalumab) in combination with etoposide and either carboplatin or cisplatin as first line treatment of patients with extensive-stage small cell lung cancer (ES-SCLC). The FDA based their approval on the results of the clinical trial CASPIAN which [...]
COVID-19 Policy Efforts – An Update From Leadership
To learn more about coronavirus and its impact on the lung cancer community, please review resources available on our coronavirus webpage. Dear Members of the Lung Cancer Community, As we continue to provide support to patients, caregivers, and providers during this public health crisis, we want you to know that [...]
Update for Week of March 23, 2020 to the Joint Statement on Coronavirus COVID-19 From Lung Cancer Advocacy Groups
As cases of the virus surge in countries around the world, with Italy being particularly hard hit, many nations are taking extreme steps to mitigate the outbreak, including whole country lockdowns. Here in the United States, the President declared a national emergency on March 13, 2020. Several states have [...]
Letter from Leadership for March 18
To learn more about coronavirus and its impact on the lung cancer community, please review resources available on our coronavirus webpage. Dear Members of the Lung Cancer Community, As we continue to monitor and respond to the rapidly evolving spread of the coronavirus (that causes [...]
Update for Week of March 16, 2020 to the Joint Statement on Coronavirus COVID-19 From Lung Cancer Advocacy Groups
The World Health Organization officially declared the COVID-19 outbreak a pandemic on March 11, 2020. As cases of the virus surge in countries around the world, with Italy being particularly hard hit, many nations are taking extreme steps to mitigate the outbreak, including whole country lockdowns. Here in the [...]
Coronavirus Response Update
Dear Members of the Lung Cancer Community, For the past several weeks, we have been closely monitoring the developments and impact around the increased spread of the coronavirus. We want each member of our community to know that your health and well-being is uppermost in our minds and hearts [...]
Statement on the passing of Amory Houghton, Jr. GO2 for Lung Cancer Board Emeritus, former Member of Congress, Chief Executive, Corning Inc.
By Laurie Fenton-Ambrose, CEO, President, and Co-Founder GO2 for Lung Cancer I share the sad news of the passing of one of GO2 for Lung Cancer’s longstanding Board members, Amory Houghton, Jr. “Amo”, as he was known by family, friends and constituents, was an admired, respected and accomplished businessman [...]
Update for Week of March 9, 2020 to the Joint Statement on Coronavirus COVID-19 from Lung Cancer Advocacy Groups
As advocacy organizations dedicated to serving the needs of lung cancer patients, all of us are closely monitoring the latest developments related to the outbreak caused by the novel coronavirus, SARS-CoV-2, and the resulting disease, COVID-19. In this update, we have included additional information on facts about COVID-19, symptoms, [...]
Joint Statement on Coronavirus COVID-19 from Lung Cancer Advocacy Groups
We understand and appreciate the severity of the new coronavirus epidemic (also known as COVID-19) that’s spreading globally. As advocacy organizations dedicated to serving the needs of lung cancer patients, all of us are closely monitoring the latest developments related to the outbreak caused by the novel coronavirus, SARS-CoV-2, [...]
Save the Date: The 2020 Lung Cancer Voices Summit
Dear Friends, This summer we will again unite the lung cancer community in Washington, DC at the 2020 Lung Cancer Voices Summit – Speaking Out as One (previously National Advocacy Summit). This summit is all about you – survivors, caregivers, advocates and providers from all around [...]
My Caregiver, My Rock
By Elizabeth de Jong Elizabeth and Sven de Jong Unlike most, we didn’t have a slow journey to my diagnosis of stage IV ALK+ non-small cell lung cancer. It was a normal Friday when I broke my femur, which led doctors to discover that I had [...]
Screening Works!
By James L. Mulshine, MD, Scientific Leadership Board, GO2 for Lung Cancer, Professor, Internal Medicine, Rush University We had another home run for the lung cancer community. On February 6, 2020 the New England Journal of Medicine published the long awaited results of the NELSON Trial (the Dutch-Belgium Lung [...]
Drug Resistance and Newly-Funded Research
by Amy C. Moore, PhD, Director, Science & Research, GO2 for Lung Cancer Patients with lung cancer face the challenge of drug resistance, while researchers focus on extending treatment duration. In collaboration with the patient group ALK Positive, we have awarded a new research collaboration grant dedicated to overcoming [...]
$500,000 Research Grant to Combat Cancer Treatment Resistance Awarded by ALK Positive and GO2 for Lung Cancer
SAN CARLOS, Calif. and WASHINGTON, Feb. 6, 2020 /PRNewswire/ -- ALK Positive (a patient-led group of 1,900+ lung cancer patients and caregivers in 50+ countries) and GO2 for Lung Cancer (a global lung cancer advocacy and education organization) are awarding a two-year, $500,000 Research Collaboration Grant to two renowned lung cancer researchers dedicated to overcoming treatment resistance. This grant funds [...]
Team Captains Baking for the Cause
Laura and Krista at their Cupcake Baking Event Meet Laura Rodriguez, a mother, survivor, and champion for the lung cancer community. When Laura was diagnosed with stage IV ALK+ lung cancer in 2012, her world forever changed. Today, she shows no evidence of disease (NED) thanks to biomarker testing and targeted therapy treatments. Laura and her [...]
Meet the Team: Susan Smedley, Lung Cancer Survivor
Susan Smedley, National Manager, Grassroots and Endurance Events At the age of 32, I was diagnosed with lung cancer. I was a new mom to my then 11-month-old daughter and my world turned upside down. After years of being misdiagnosed with asthma and chronic fatigue, I decided [...]
The Power of the Patient Voice
Patient-reported outcomes (PROs) have been used in cancer clinical trials for more than 20 years. Along with clinical evidence, healthcare decision-makers use PROs to guide both individual patient choice and shared decision making. Unfortunately, the patient voice is often lost in clinical trial development because of inadequate or poor [...]
Lung Cancer Research: How You Can Make a Difference
Did you know that you can help change the future of lung cancer? One of GO2 for Lung Cancer’s core pillars is to advance world-class, patient-centered, academic and community-based research that spans the continuum of care. To achieve this we, along with our partners and collaborators, offer our community [...]
3 Ways to Support the Lung Cancer Community in 2020
We’re excited to be starting off our first full calendar year as GO2 for Lung Cancer. There is so much to look forward to this year. From the programs you know and love, to advocacy on Capitol Hill, this will be a huge year for the entire lung cancer [...]
The Lung Cancer Living Room: Q&A with Danielle Hicks, Chief Patient Officer
Every month our Lung Cancer Living Room, a patient education and support series, brings hope home to patients and their families around the world. Through presentations by experts in the field, this unrestricted forum covers all topics, from early detection to personalized medicine and beyond. As the first lung [...]
Advances in Lung Cancer Treatment Drops U.S. Cancer Deaths
GO2 for Lung Cancer points to targeted treatments and early screenings SAN CARLOS, Calif. and WASHINGTON, Jan. 8, 2020 /PRNewswire/ -- Today's report concerning improvements in cancer mortality is welcomed news. The recently released Cancer Statistics 2020 report shows that the decline in lung cancer deaths is driving the decrease in mortality rates for cancer overall. [...]
Year-end Progress for the Lung Cancer Community: Federal Research Funding & the Women and Lung Cancer Research and Preventive Services Act
For many weeks, our community has been awaiting passage of the Fiscal Year 2020 (FY20) federal budget, which funds the Lung Cancer Research Program. This week, just days before the budget continuing resolution expired, the House and Senate released the long-awaited conferenced agreement that has been signed by the [...]
Stories That Inspire
From advancements in research and progress on Capitol Hill, to working directly with survivors and families, 2019 has been a monumental inaugural year for us at GO2 for Lung Cancer. At the heart of everything we do is our patient community; you continue to inspire and motivate us every [...]
Lung Cancer Registry Launches New Surveys
SAN CARLOS, Calif. and WASHINGTON DC, December 12, 2019 – The Lung Cancer Registry has launched new surveys to do an even better job of capturing important moments in a patient’s lung cancer journey. These new surveys include questions about how symptoms and side effect impact quality of life—now [...]
Share the Life-Saving News About Lung Cancer Screening
Help raise awareness of the importance of lung cancer screening! Just like mammograms for breast cancer screening and colonoscopies for colon cancer screening, a test is now available that can find and detect lung cancer early, which is the leading cause of cancer death worldwide for both men and [...]
FDA Approves Tecentriq Plus Chemotherapy as a First-Line Treatment for Metastatic NSCLC
On December 3, 2019, the U.S. Food and Drug Administration approved Tecentriq (atezolizumab) in combination with chemotherapy (Abraxane [paclitaxel protein-bound; nab-paclitaxel] and carboplatin) as a first treatment for patients with metastatic non-squamous non-small cell lung cancer (NSCLC) with no EGFR or ALK changes. This approval is based on the [...]
GO2 for Lung Cancer Announces Young Innovators Team Award Recipients
SAN CARLOS, Calif., Dec. 3, 2019 -- GO2 for Lung Cancer announced the recipients of the 2019 Young Innovators Team Awards (YITA) today. Two teams of researchers received grants totaling $500,000 for their work developing novel strategies to treat KRAS-mutant non-small cell lung cancer (NSCLC) and examining the impact of age on development, progression, and [...]
The Power of Partnerships—EGFR-Mutant Lung Cancer
We fight lung cancer together. That’s a lesson we’ve learned and put into action. Amy C. Moore, PhD, our Director of Science & Research, recently published an article in the International Association for the Study of Lung Cancer online news about the importance of building research models utilizing partnerships [...]
“Simply the Best” Dinner and Gala Honors Those Working to Fight Lung Cancer
AstraZeneca Announced as 2019 "Simply the Best Award" Honoree SAN CARLOS, Calif. and WASHINGTON DC, November 12, 2019 – GO2 for Lung Cancer (a merger of the Bonnie J. Addario Lung Cancer Foundation and Lung Cancer Alliance) recognized AstraZeneca on November 9 at its 14th annual "Simply the Best" [...]
LCAM 2019: Easy Ways to Make a Big Difference
We’re just under one week into Lung Cancer Awareness Month (LCAM) 2019! This November, we’re making it easy to turn your passion into progress with a different action for each day on our LCAM Action Center. Some are big and some are small – but every single effort made [...]
It’s Time to Shine!
Can you believe Lung Cancer Awareness Month kicks off tomorrow? Our Shine a Light on Lung Cancer events make it easy for you to celebrate and remember survivors, caregivers and all individuals who have been impacted by the disease. What can you expect at a Shine a Light event? [...]
A Reason to Run: Chris’s Story
Chris Palumbo, born and raised in Rochester, New York, is often found running races and participating in endurance challenges in his home city and beyond. He carries the GO2 for Lung Cancer flag with him in support of his best friend Jeff Clark, who was diagnosed with stage IV [...]
Be a Champion This Lung Cancer Awareness Month!
We’re just a few short weeks away from the start of Lung Cancer Awareness Month (LCAM)! This is a critical time of year for the lung cancer community as we unite to raise awareness and funds, educate, and spread hope. There are countless opportunities for you to step up [...]
A Caregiver’s View
By Danielle Hicks, Chief Patient Officer, GO2 for Lung Cancer I became a caregiver when my mom was diagnosed with stage 3B lung cancer in 2003. It was one of most terrifying things I had ever had to face up until that point in my life. She was only [...]
Top Science from the 2019 World Conference on Lung Cancer
This week, the GO2 for Lung Cancer team attended IASLC’s 20th Annual World Conference on Lung Cancer in Barcelona. Our team presented on hot topics in the field alongside top clinicians, researchers, and scientists from over 100 countries. We sat down with Jennifer C. King, PhD, Senior Director of [...]
Know Your Nodules
Decoding lung cancer terminology can be tough—but we’re here to make it easier. In this blog, we’ll help you better understand nodules, also known as lesions, coin lesions, growths, or solitary pulmonary nodules. Nodules are abnormal (yet common) spots that may show up on your lung cancer screening scan [...]
Quality of Life
Isn’t that what it’s all about? Whether a CT scan detects lung cancer early or treatment navigation helps you receive biomarker testing or a HelpLine staff member virtually holds a hand, enhancing quality of life for you and all the lung cancer community is of paramount importance. Support can be [...]
Pedaling for Lung Cancer Awareness
How far will someone go to raise awareness about lung cancer? Try 1,800 miles. That’s how far the participants in the Ride Hard Breathe Easy Classic (RHBE) will pedal to recognize the need for enhanced visibility and funding, and to offer hope for future treatments for lung cancer. Started [...]
Welcome to Belong, a New Online Cancer Community
GO2 for Lung Cancer has partnered with Belong.Life to provide patient and caregiver support and education on the world’s largest social network for cancer patients. Within the GO2 Foundation membership area, our social workers, clinical trial specialists, and survivor-volunteers will provide hope, education, and support for those living with lung [...]
FDA Approves Genentech’s Rozlytrek (entrectinib) for ROS1 Non-Small Cell Lung Cancer (NSCLC) and NTRK-Driven Tumors
On August 15, 2019 the U.S. Food and Drug Administration approved Rozlytrek (entrectinib) for the treatment of patients with ROS1-positive, metastatic non-small cell lung cancer. It was also simultaneously approved for the treatment of patients with any solid tumor that is NTRK fusion-positive, following progression on other therapies. This [...]
Celebrating Survivorship and Making a Difference
Don Stranathan, well-known in the lung cancer community, sometimes is referred to as Superman or Dr. Don. He is well-informed, tenacious, kind, and is always happy to share what he knows with other lung cancer patients. In 2009, he was diagnosed with stage 4 lung cancer. Survivors created [...]
Crisis and Relief: Reflections on World Lung Cancer Day
World Lung Cancer Day is a time to remember that this disease remains a crisis around the globe. But is also a day to count the many ways that we’ve helped advance efforts on general awareness, early detection, research funding, innovative research, and support for patients and caregivers. Providing [...]
GO2 for Lung Cancer Presents 2019 Bonnie J. Addario Lectureship Award at ILCC
GO2 for Lung Cancer is pleased to announce that it presented Solange Peters, MD, PhD, with the 2019 Bonnie J. Addario Lectureship Award for her emphasis on groundbreaking biomarker research, improving access to care for lung cancer patients worldwide, and supporting the advancement of women in oncology. GO2 Foundation honored Prof. [...]
An Update on the Burn Pits Accountability Act of 2019
Click here to read our statement in support of the legislation. GO2 for Lung Cancer is pleased to report the recent advancement of the Burn Pits Accountability Act. We strongly support this legislation as a first step to ensure that all those affected by burn pit toxins are accounted [...]
Make an Impact from Home, 7/23!
On July 23, over 130 advocates will meet with elected officials on Capitol Hill to advocate for the lung cancer community at the GO2 for Lung Cancer National Advocacy Summit. Now you have the opportunity to strengthen the collective voice and ignite change—without leaving your home! Join us on [...]
Lung Cancer Stigma’s Harmful Consequences
Despite increased awareness around lung cancer in recent years, the stigma associated with the disease remains an issue for the lung cancer community. Beyond the known psychosocial issues associated with this stigma, our research has revealed that both patients and oncologists feel that the stigma can actually affect the level of [...]
Going the Extra Mile: Jason’s Story
Losing a loved one to lung cancer is a pain that no one should have to experience. After Jason’s mother passed away from lung cancer in May 2018, he committed to doing something positive in her honor. On November 3, he’ll be running in the 2019 New York City Marathon [...]
Patient Needs Represented on The Hill
This week, The Hill hosted an event on Cost, Quality and Care: The Medicare Equation, where experts gathered to discuss the balancing act between Medicare affordability and quality of care. Our Co-founder, President, and CEO, Laurie Fenton Ambrose, brought the patient voice to the discussion.“Just addressing the cost of drugs is [...]
New Treatment Approved for Metastatic Small Cell Lung Cancer
On June 17, 2019, the U.S. Food and Drug Administration granted accelerated approval to pembrolizumab (KEYTRUDA) for patients with metastatic small cell lung cancer (SCLC) with disease progression on or after platinum-based chemotherapy and at least one other prior line of therapy. Though pembrolizumab is an established treatment option [...]
Early Screening Equals Early Diagnosis
GO2 for Lung Cancer’s recently published scientific paper found that lung cancer screening programs can successfully detect lung cancer early with the majority of lung cancers diagnosed at stage 1 or limited stage. Importantly, outcomes at academic and nonacademic sites were compared and the data confirmed that responsible screening [...]
Lung Cancer News from the Largest Oncology Meeting
The GO2 Science and Research team just returned from the 2019 American Society of Clinical Oncology (ASCO) meeting. We connect you and your loved ones to the latest research and treatment options by keeping up with the latest advances at meetings like ASCO. While there we saw exciting presentations [...]
Research Wins Big
The GO2 for Lung Cancer is honored to announce that on May 2 two of our programs received Your Cancer’s Cancer Community (C2) Awards. These awards celebrate “the unsung heroes of cancer care” – individuals and organizations that advance cancer care and work to improve the lives of those [...]
Top Five Tips When Seeking Financial Assistance on Your Lung Cancer Journey
If you or a loved one have lung cancer, you know that it is not only taxing emotionally but also financially. And it’s not just the high prices of medication that take a toll on your wallet, it’s the gas needed to fill your car for the multiple hour [...]
Lung Cancer Caucus Reestablished for 2019
Tuesday, March 26, 2019 — Today, Congressman Brendan F. Boyle (D-PA-02) and Congressman John Rutherford (R-FL-04) announced that they are serving as Co-Chairs of the Congressional Lung Cancer Caucus in the new 116th Congress. A big thank you from Lung Cancer […]
Register for the UNITED Portland Run/Walk, June 22!
This year, for the first time in Portland, two of the leading lung cancer organizations are joining forces to create one of the largest run/walk events for lung cancer on the west coast…because together we are stronger. Join Lung Cancer Alliance and Bonnie J. Addario Lung Cancer Foundation at Laurelhurst Park on Saturday, [...]
Our First Cancer Support Group Meeting
By Ginny Hicks My friend and stage IV lung cancer survivor, Rick Rose, and I decided to start a lung cancer support group in Medford, Oregon. We contacted Lung Cancer Alliance for guidance and we were provided with a helpful manual with great ideas for procedures, topics and troubleshooting. [...]
Lung Cancer Screening: What to Expect
Lung cancer screening. You may have seen it on the news or an advertisement for it, but what is it and does it make sense for you? Screening refers to a test for individuals considered to be at high risk for a disease before they have symptoms. Lung cancer [...]
The Hardest Times Require the Strongest Support
By Erik Hale When I was just 30 years old I was diagnosed with stage 3A adenocarcinoma, non-small cell lung cancer. After diagnosis I spent six weeks going through radiation and chemotherapy treatment to reduce the tumor size before going in for a lobectomy procedure where half of my left lung [...]
Traveling with Cancer
By Elizabeth De Jong, lung cancer survivor My parents had an (at the time) unconventional commuting marriage for most of my childhood. Weekends were spent in cars visiting the parent that wasn’t living with us at the moment. These trips sparked a wanderlust in me at a young age. [...]
What a Difference a Year Makes
Dear Lung Cancer Community, As we dive into 2019 and further our efforts to advance new life-saving research and treatments for the lung cancer community, we want to take a moment to say thank you and reflect on what we accomplished together in 2018. Distributed over 90,000 pieces of [...]
2019 Forecast for Lung Cancer Research and Treatments
Last year was an exciting year for lung cancer research with more U.S. Food and Drug Administration (FDA) approval decisions relevant to lung cancer than ever before. So, what can we expect for 2019? Another eventful year with researchers studying how to best give the approved drugs (i.e. what [...]
LCA Joins ACS CAN in Efforts to Protect Patient Access to Lifesaving Treatment
Thursday, January 17 – Lung Cancer Alliance joins a long list of leading patient advocacy groups in support of American Cancer Society Cancer Action Network’s (ASC CAN) advertising campaign, urging the U.S. Administration and Congress not to limit patient access to lifesaving medications covered under Medicare, and to ensure [...]
Don’t Guess, Test!
By Kimberly Buchmeier My name is Kimberly Buchmeier and I am from a small town in Southeast Nebraska. I am a mother, wife, grandmother, daughter, sister, aunt, cousin, friend and a six-year lung cancer survivor. In 2011, at age 37, I was diagnosed with non-small cell adenocarcinoma. I [...]
New Year, New You!
Goodbye 2018, hello 2019! With the new year comes new opportunity to recommit to your health and well-being. If you’re part of the 41% of Americans looking to set a New Year resolution, we can help! Here are four worthy commitments to consider this year. FIND SUPPORT: Support in your [...]
Get Well Soon Justice Ginsburg
On behalf of the lung cancer community we serve, we send our best wishes for a speedy recovery to Supreme Court Justice Ruth Bader Ginsburg. According to a Supreme Court press release, Justice Ginsburg is now resting comfortably after undergoing a pulmonary lobectomy today. Two nodules, found to be [...]
A Message from LCA’s President
To the Lung Cancer Community, As 2018 prepares to come to a close, let’s take this moment to reflect upon what we have accomplished to improve survivorship, provide support and bring our community together in a lasting and meaningful way. Together we secured millions more dollars in federal research [...]
Happy Holidays!
https://www.youtube.com/watch?v=kO8_l3xrASY
Giving—Why Not?
With the season of giving upon us, now is the perfect time to join the fight against lung cancer. Over 234,000 people were diagnosed with lung cancer this year, making it the leading cause of cancer death for both men and women across all ethnicities. You can help us [...]
New Combination Treatment Approved for Non-Small Cell Lung Cancer
The Food and Drug Administration (FDA) approved atezolizumab (TECENTRIQ®), an immunotherapy drug, in combination with bevacizumab, paclitaxel, and carboplatin for the first-line treatment of patients with metastatic non-squamous, non-small cell lung cancer (NSq NSCLC) with no EGFR or ALK genomic tumor aberrations. This is the first four drug combination [...]
LCA partners with GRAIL, UCL, and ULCH on Newly Announced SUMMIT Study
Monday, December 3, 2018 –Today, Lung Cancer Alliance, in collaboration with GRAIL, Inc., a healthcare company focused on the early detection of cancer, UCL (University College London) and University College London Hospitals National Health Service (NHS) Foundation Trust (UCLH) announced plans to initiate the SUMMIT study in early 2019. [...]
4 Simple Steps to Finding the Right Clinical Trial
Today, there are more options for lung cancer treatment than ever before. Some are already approved and available. Others are only available through clinical trials. With so many clinical trials out there, the process of finding one can seem daunting. Not to worry! We have you covered. Here are [...]
FDA Approves Targeted Therapy for Solid Tumors with NTRK Gene
Tuesday, November 27, 2018 - The U.S. Food and Drug Administration (FDA) granted accelerated approval to larotrectinib (VITRAKVI, Loxo Oncology Inc. and Bayer) for adult and pediatric patients with advanced solid tumors, including lung cancer, that have a neurotrophic receptor tyrosine kinase (NTRK) gene fusion without a known resistance [...]
Honor and Salute the Veterans In Your Life
By Rick Sherlock, Lung Cancer Alliance Board Member and retired Major General of the United States Army On this Veteran’s Day, we honor and salute the dedicated service of the brave men and women that protect our freedoms. In all they do for our country, it is our responsibility [...]
5 Tips to Help Your Loved One with Lung Cancer
When a loved one finds out they have lung cancer it can be hard to know how best to help them, what to say or even how they are feeling. Here are five helpful tips for being the best caregiver to your loved one. Take care of yourself. There [...]
2018 Lung Cancer Support Group Facilitator Award
Monday, November 5, 2018 -- Today, Lung Cancer Alliance presented the 2018 Lung Cancer Support Group Facilitator Award to Catherine Paykin, LCSW-R, who runs a support group at New York University Perlmutter Cancer Center. The Lung Cancer Support Group Facilitator Award recognizes the uncommon dedication of a support group facilitator. Members [...]
FDA Approves New Treatment for ALK+ NSCLC Patients
Friday, November 2, 2018 – Today the Food and Drug Administration (FDA) granted accelerated approval to lorlatinib (LORBRENA) for patients with anaplastic lymphoma kinase (ALK)-positive metastatic non-small cell lung cancer (NSCLC) whose disease has progressed on crizotinib and at least one other ALK inhibitor for metastatic disease or whose disease [...]
LCA Named 2018 Top Rated Nonprofit
Friday, November 2, 2018 - Today Lung Cancer Alliance was named a “2018 Top-Rated Nonprofit” by GreatNonprofits, the leading provider of user reviews of charities and nonprofits. The Top-Rated Nonprofit Award is based on the rating and number of reviews that Lung Cancer Alliance received from volunteers, donors and aid [...]
LCA Partners with UAB Comprehensive Cancer Center to Help At-Risk, Underserved Communities
Washington, D.C. - November 1, 2018 –Lung Cancer Alliance (LCA) today announced a $1.6M grant from the Bristol-Myers Squibb Foundation to partner with the University of Alabama at Birmingham (UAB) Comprehensive Cancer Center to establish the Alabama Lung Cancer Awareness, Screening and Education (ALCASE) program. The three-year effort will [...]
Lung Love Walk Houston: Support Tami’s Titans!
Lung Love Walk Houston is this weekend! Join us on Saturday, November 3, 2018 at the Buffalo Bayou Park in Houston, TX to support Tami's Titans and all the other teams walking/running in the fight against lung cancer. We can't wait to see you there!
New Directions in Small Cell Lung Cancer
More updates have been made in small cell lung cancer (SCLC) research and treatment since this blog was originally published. Explore our small cell lung cancer blog feed to learn more. Small cell lung cancer (SCLC) makes up about 15% of lung cancer cases (the other 85% is [...]
Lung Cancer Awareness Month Kick-Off!
Easy Ways to Get Involved Today kicks off November, Lung Cancer Awareness Month, the time of year when our community comes together to raise awareness, educate and spread hope. There are lots of ways to get involved and make a difference. Throughout the month we will be providing you [...]
LCA Launches Nationwide Screening Awareness Campaign
“What About Your Lungs?” Campaign Urges Individuals At-Risk for Lung Cancer to Talk to Their Doctor About Life-Saving Screening Washington, DC [Wednesday, October 31, 2018]—Responding to the need to increase national awareness about lung cancer screening and encourage those at risk to talk with their doctor, Lung Cancer Alliance [...]
FDA Approves New Combination Treatment for NSCLC
Tuesday, October 30, 2018 – Today the Food and Drug Administration (FDA) approved immunotherapy drug, pembrolizumab, also known as KEYTRUDA, in combination with chemotherapies, carboplatin, and either paclitaxel or nab-paclitaxel as a first-line treatment for patients with metastatic squamous non-small cell lung cancer (NSCLC). The results of the randomized [...]
6 Ways to Make a Difference this November
Lung Cancer Awareness Month is fast approaching with just two weeks until November! This is the time of year when our community comes together to raise awareness, educate and spread hope. There are lots of ways to get involved and make a difference. See which one makes sense for [...]
LCA Urges CMS to Remove Barriers to Screening
In a number of communications to the Centers for Medicare and Medicaid Services (CMS), Lung Cancer Alliance urged the national CMS office to 1) instruct all local Medicare contractors that lung cancer screening coverage is available in all settings including Independent Diagnostic Testing Facilities across the country and 2) [...]
Now Is the Time for Advocacy At Home!
By Elridge Proctor, MPA, Director of Health Policy We have come a long way, but still have far more work to do in raising awareness about the profound impact that lung cancer has on Americans in every state across the nation. November is Lung Cancer Awareness Month and mid-term elections. [...]
FREE WEBINAR: Small Cell Lung Cancer
WEBINAR: New Directions in Small Cell Lung Cancer Thursday, November 1, 2018 from 12:00PM- 1:00PM Do you or a loved one have small cell lung cancer and are looking for information on the latest treatments and advancements? Join us for a webinar on November 1 at 12:00PM EST [...]
Doing My Part
By Michelle McMahon My connection to lung cancer began when I was a teenager. I lost my mom to this disease just before her 53rd birthday. Some 33 years later I was handed the same lung cancer diagnosis in the form of Bronchoalveolar Carcinoma (BAC). I was 49 years [...]
New First-Line Treatment Approved for NSCLC with an EGFR Mutation
Friday, September 28, 2018 – Today the Food and Drug Administration approved VIZIMPRO tablets (dacomitinib) for the first-line treatment of patients with metastatic non-small cell lung cancer (NSCLC) with an EGFR (epidermal growth factor receptor) mutation. Approval was based on a randomized trial that showed a significant improvement in [...]
Highlights from World Conference on Lung Cancer
This week, the LCA team attended the 19th Annual World Conference on Lung Cancer (September 23-26, 2018) in Toronto, Canada. Jennifer C. King, PhD, Director of Science and Research, gives us the scoop on some of the research and treatment breakthroughs, and how they impact you! https://youtu.be/Q2S7akrHUVQ SCREENING REDUCES [...]
Passage of Historic Appropriations Bill Cements Funding for Lung Cancer Research
Cancer and Health Programs See Gains in FY19 Budget Wednesday, September 26, 2018 – Today, Lung Cancer Alliance (LCA) acknowledges the historic passage of the combined Department of Defense (DOD) and Labor, Health and Human Services (LHHS) appropriations package by the House following approval by the Senate. The bill [...]
Trial Results Show Screening Significantly Reduces Lung Cancer Mortality Rates
Washington, DC [September 25, 2018] – Lung Cancer Alliance (LCA) applauds the results of the Dutch-Belgium Lung Cancer Screening trial (NELSON) announced today in Toronto, Canada at the IASLC 19th World Conference on Lung Cancer. The NELSON study was a population-based, controlled trial that enrolled 15,792 individuals, who were [...]
Lung Cancer Stigma: Where Are We Now?
Lisa Carter-Harris, PhD, APRN, ANP-C By Lisa Carter-Harris, PhD, APRN, ANP-C, Indiana University School of Nursing, Indianapolis, IN This year, I joined Lung Cancer Alliance in replicating a national survey, that was first done in 2008, to examine the perspectives of patients, oncologists and the general public on [...]
LCA Survey Results Show Unmet Emotional Needs of Long-Term Survivors
Sunday, September 23, 2018 -- Lung Cancer Alliance's Director of Support Initiatives, Maureen Rigney, LICSW presented findings from a survey done on long-term lung cancer survivors at the official press conference at the 19th Annual World Conference on Lung Cancer in Toronto, Canada. Online survey data showed lung cancer survivors [...]
LCA’s Research Showcased on a World Stage
The Lung Cancer Alliance team is heading to IASLC's World Conference on Lung Cancer (WCLC), Toronto, Canada from September 23-28, 2018 and will be presenting on hot topics in the field of lung cancer including: clinical trials, long-term survivorship, stigma, advocacy and much more! Below are details on our whereabouts [...]
An Unexpected Path
Shanen M. Sherrer, PhD By Shanen M. Sherrer, PhD Shanen M. Sherrer, PhD, is the Assistant Professor of Biochemistry, Department of Chemistry and Biochemistry, St. Mary’s College of Maryland. We interviewed Dr. Sherrer to find out about her career as a cancer researcher, how attending a Shine [...]
Survey Shows That Despite Gains, Stigma Remains a Problem
SURVEY SHOWS INCREASED PUBLIC AWARENESS OF LUNG CANCER OVER LAST DECADE Despite Gains, Stigma Remains a Problem; Survivors Continue to Shoulder Blame for Diagnosis Wednesday, September 5, 2018 – Survey results released today by Lung Cancer Alliance (LCA) show that general awareness about lung cancer has improved significantly over the last [...]
Tribute to Senator John McCain
Lung Cancer Alliance (LCA) joins the millions of Americans and others from around the world in remembering the extraordinary life of Senator John McCain (R-AZ) who passed away from brain cancer on August 25, 2018. He was a true American hero who embodied values that we admire and seek [...]
Immunotherapy Comes to Small Cell Lung Cancer
By: Jennifer C. King, PhD, Director of Science and Research, Lung Cancer Alliance Last week, the United States Food and Drug Administration (FDA) approved Opdivo (nivolumab) as a new treatment for small cell lung cancer. This is the first new drug for small cell lung cancer that has been [...]
Make a Difference TODAY – 8/20!
Advocacy Action Day — August 20, 2018 Since our National Advocacy Summit on July 10-12, where over 120 lung cancer advocates joined forces in Washington, DC to meet with their elected official and demand that lung cancer be recognized as a national priority, we have seen nearly 50 representatives [...]
First New Treatment Approved for Small Cell Lung Cancer in 20 Years
Friday, August 17, 2018 – Today the U.S. Food and Drug Administration approved Opdivo (nivolumab), an immunotherapy treatment, for patients with metastatic small cell lung cancer (SCLC) with progression after platinum-based chemotherapy and at least one other line of therapy. Accelerated approval was granted for this therapy due to [...]
Palliative Care: Managing Symptoms and Improving Quality of Life
We partnered with GetPalliativeCare.org and Dr. Andrew Esch, MD, MBA, a medical education consultant to the Center to Advance Palliative Care, for a free webinar discussing how to manage symptoms and side effects of lung cancer and improve quality of life through palliative care. Here’s what you need to know! [...]
LCA Advocates for Patient Access to Robotic Radiosurgery (Cyberknife)
Last month, Lung Cancer Alliance (LCA) submitted detailed comments to Noridian Healthcare Solutions, a Medicare contractor, regarding the unintended consequences and patient access implications to reductions in healthcare reimbursement for CyberKnife, robotic radiosurgery/stereotactic body radiation. In response to LCA’s comment letter and other stakeholder feedback, Noridian modified their policy [...]
Managing Lung Cancer Symptoms and Improving Your Quality of Life
Dr. Andrew Esch, MD, MBA Dr. Andrew Esch, MD, MBA Dr. Esch is a medical education consultant to the Center to Advance Palliative Care. A palliative care specialist, Dr. Esch focuses on improving the quality of life for patients and their families as they face serious illness. [...]
A Milestone Summit (in more ways than one)!
The 10th Anniversary National Advocacy Summit will be remembered as one of the most impactful and memorable gatherings of the lung cancer community to date. Not only did we have a record number of lung cancer survivors represented (nearly half of the 120 attendees!), but we also received more [...]
LCA Opens Milestone 10th Anniversary National Advocacy Summit
More than 120 Advocates from 28 States Bring Their Collective Voice to Washington, DC Washington, DC [July 10, 2018] – Today, Lung Cancer Alliance (LCA) opens the 10th Anniversary National Advocacy Summit in Washington, DC. Taking place from July 10 –12, the Summit will bring together 120 survivors, loved [...]
LCA Commends GLCC for Recognizing Excellence in Journalism Coverage of Lung Cancer
Monday, July 9, 2018 - Today, Lung Cancer Alliance (LCA) is proud to acknowledge the Global Lung Cancer Coalition (GLCC) for selecting the winners of the 2017 Global Lung Cancer Coalition Journalism awards. Each year, the GLCC recognizes outstanding journalistic efforts to advance awareness of lung cancer, the number [...]
Lung Cancer Alliance Joins CURE Media Group Strategic Alliance Partnership (SAP) Program
Monday, July 2, 2018 - Today, CURE Media Group announced that LCA has joined its Strategic Alliance Partnership (SAP) Program. The SAP Program is designed to facilitate an open exchange of information among trusted peers, with the goal to improve patient care. The content sharing collaboration will empower the [...]
$20M Secured For Federally-Funded Lung Cancer Research
House Passes Bipartisan Amendment to Increase Funding in FY19 Budget; Restores Funding to FY09 Level Thursday, June 28, 2018 — Today, Lung Cancer Alliance hailed the passage of a bipartisan amendment in the U.S. House of Representatives that secured an additional $6 million to the Department of Defense (DOD) [...]
Meet Anita! Our Screening Expert
By Anita McGlothlin, Associate Director of Science and Regulatory Policy, Lung Cancer Alliance Greetings! I am Anita McGlothlin, the Associate Director of Science and Regulatory Policy at Lung Cancer Alliance (LCA). I can’t wait to meet you in person at a future LCA event. In the interim, I’m excited [...]
Host a Shine a Light on Lung Cancer Event this November!
It's that time of year again! Time to start planning your Shine a Light on Lung Cancer event! This year marks the 10th Annual Shine a Light on Lung Cancer event! With Shine a Light, healthcare facilities celebrate and remember survivors, caregivers and all individuals who have been impacted by [...]
My Difference Maker
Patty (third from right) and her family By Patty Watkins I learned that I had stage 4 adenocarcinoma in February 2014 and was only given two days to live, but fortunately survived beyond this bleak, initial diagnosis. In May 2014 I had my tumor tissue tested for [...]
My Most Important Job
David and his son, Connor. By David Marshall Four years ago I was told I had stage 4 lung cancer. I remember the day well. At the time, I was 40 years old, in great shape and very energetic. I had developed a horrible cough and a [...]
Survivor Advice for the Newly Diagnosed
We asked the lung cancer community the following question and here is what they said. "What is one piece of advice you would offer someone newly diagnosed with the disease?" "Don't Google lung cancer statistics! Listen to your body, rest when you're tired. Accept help from family and friends. [...]
Lung Cancer Advocacy: Why It Matters to Me
During the more than three years since our youngest daughter, Katherine, was diagnosed with Stage 4 non-small cell lung cancer, we have joined forces to advocate for more funding for lifesaving research into why so many young women get lung cancer every year. Here we are at last [...]
Expanding the Reach of Precision Medicine
By Jennifer C. King, PhD, Director of Science and Research at Lung Cancer Alliance Expanding the Reach of Precision Medicine Lung cancer research was everywhere at the American Society of Clinical Oncology (ASCO) conference held June 1-5. The ASCO President, Bruce E. Johnson, MD, contrasted this meeting with the [...]
Our Impressions from ASCO 2018
https://youtu.be/zGCowDzDwPM
LCA Supports Research to Treatment Pathways for Small Cell Lung Cancer
Monday, June 4, 2018 --Today, Lung Cancer Alliance (LCA) is proud to announce Carl M. Gay, MD, PhD as the recipient of the 2018 Conquer Cancer Foundation of ASCO/Lung Cancer Alliance Young Investigator Award (YIA). Through this award, LCA will support Dr. Gay’s research into whether inhibiting DNA repair [...]
LCA Remains Vigilant in Advocating for At-Risk Individuals
Wednesday, May 30, 2018 – Lung Cancer Alliance (LCA) continues to represent those at risk for lung cancer and has reignited coalition efforts through a partnership with the Society of Thoracic Surgeons (STS) and American College of Radiology (ACR). The coalition submitted recommendations to United States Preventive Services Task [...]
My Mom is a Lung Cancer Survivor
By Congresswoman Suzanne Bonamici (D-OR-01), Member of the Congressional Lung Cancer Caucus As a policymaker, I know we must do more to prevent lung cancer and support patients – especially women – who are battling this devastating disease. I’m proud to help lead the bipartisan Women and Lung Cancer Research [...]
News Flash: Our Lungs Matter And Screening Does Save Lives
By Laurie Fenton Ambrose, President and CEO, Lung Cancer Alliance Make no mistake about it—lung cancer can be detected at an early stage, when it is more likely to be treatable and cured. Yes, you heard it right—treatable and cured. And not just for a handful of people, but [...]
Congress: When Considering Women’s Health, Don’t Forget about Lung Cancer
An Op-Ed on Women and Lung Cancer recently ran in The Hill, a top policy newspaper and newsite read by national legislators. The opinion piece was co-authored by Laurie Fenton Ambrose, President & CEO of Lung Cancer Alliance and Bonnie J. Addario, founder and chair of the Bonnie J. Addario Lung Cancer [...]
Clinical Trial Myths Busted!
Clinical trials are an important consideration in your lung cancer treatment path, but finding the right one can be overwhelming. Thanks to our Clinical Trial Matching Service partners, Antidote, we busted some of the five most common myths about clinical trials. Myth #1: I can’t take part in a clinical [...]
A Champion for Lung Cancer Screening
Cecelia Brewington, MD is a board-certified practicing radiologist, radiology administrator and professor who serves as Medical Director of UT Southwestern Medical Center’s CT Lung Cancer Screening Program; a program she helped found. We interviewed Dr. Brewington to find out more about her 25 year career, what she wants people to [...]
Lessons My Mom Taught Me Battling Lung Cancer
Maddie (right) and her mom, Dawn (left). By Maddie Horner “Warrior,” “rock star,” “incredible,” “amazing,” “strong” and “inspiration” are all words people have used to describe my mom. To me, she is those things and so much more. She has always been the rock in my family, [...]
New UNITED Philadelphia Run/Walk at the Zoo!
This year, for the first time ever, two of the leading lung cancer organizations are joining forces to create one of the largest run/walk events for lung cancer on the east coast…because together we are stronger. Join Bonnie J. Addario Lung Cancer Foundation and Lung Cancer Alliance at the [...]
What Type of Volunteer Are You?
It’s not a one-size-fits-all approach when it comes to getting involved in the fight against lung cancer. Below are the four most popular types of volunteers we see and opportunities that apply to each. What kind are you? The Newbie There is never a bad time to join the [...]
Why I’m A Lung Cancer Advocate
Hugo and his wife. By Hugo Rizzo You never really know how devastating an illness can be until it touches your own life. That’s what I learned on March 20, 2015, my brother’s birthday, when the celebration of that milestone was forever changed with the words, “You [...]
A Tribute to Jane Reese-Coulbourne, LCA Honorary Board Member
Jane Reese-Coulbourne By Laurie Fenton Ambrose, President & CEO of Lung Cancer Alliance My heart is heavy thinking about a world without Jane Reese-Coulbourne – an extraordinary woman, leader, advocate, and friend – who passed this week from cancer. Her death leaves a gaping hole in the [...]
Commit to Clean Air with these Simple Changes
Each day we take about 23,040 breaths! Needless to say, the quality of the air we breathe is very important. Pollution of our air comes in many forms from vehicle and diesel exhaust to industrial and residential emissions. Unfortunately, these substances contaminate the air and can lead to long-term [...]
The New Frontier: Immunotherapy Combinations
At the annual American Association of Cancer Research (AACR) conference, scientists presented new research on what they called “The New Frontier in Lung Cancer” – immunotherapy combinations. The Big Question: Do Combinations Work? If we take the new immunotherapy drugs (like Keytruda, Opdivo, or Tecentriq) that have shown positive [...]
FDA Approves Tagrisso as First-line Treatment for NSCLC
Thursday, April 19, 2018 – This week the Food and Drug Administration (FDA) approved targeted therapy drug, osimertinib (Tagrisso), as a frontline treatment for patients with non-small cell lung cancer (NSCLC) who have tumors with the EGFR mutations. The approval is based off results from a study showing that [...]
Grieving a Cancer Diagnosis and How to Deal
By Trudy Lechner, RN, BA, CDE, Medical and Bereavement Counselor Trudy is a Registered Nurse and Bereavement Counselor who has worked in the field of Death and Dying for 26 years. The theory referenced below was established by Dr. Elisabeth Kübler-Ross. I am a lung cancer survivor. I was [...]
Living For My Dad
By Rachel Heimler Richard (second from left), Rachel (third from right) and their family. Today would have been the 14th anniversary since my dad, Richard, was diagnosed with lung cancer. Each year, for the past 14 years, we have woken up to a letter from my dad [...]
How I Got My Second Wind… and Wrote a Book About It
By Dann Wonser How many things have you learned since your cancer journey began, that you wished you had known right from the beginning? If you’re like me, it’s a long list. How do you get through the shock of those first few weeks? (Umm… months?) How do you [...]
My Public Health Path to Lung Cancer
By Amy Copeland, MPH, Director of Medical Outreach at Lung Cancer Alliance Public Health Week (April 2-9) got us thinking….What exactly is public health and how does it relate to lung cancer? We sat down with Amy Copeland, Lung Cancer Alliance’s (LCA) Director of Medical Outreach, who received her [...]
Reflections from the Front Lines
By Eric H Bernicker, MD - Assistant Professor of Clinical Medicine in Oncology, Institute for Academic Medicine, Assistant Clinical Member, Research Institute, Houston Methodist If you could bring a lung cancer researcher from the 1970s in a time machine to see our present treatment landscape, they would be truly dumbfounded. [...]
Additional $14 Million Secured For Federally-Funded Lung Cancer Research
Monday, March 26, 2018 — Today, Lung Cancer Alliance is pleased to acknowledge last week’s passage of the Omnibus FY18 Appropriations Bill by Congress. After months of operating under a continuing resolution, The President signed the $1.3 trillion dollar bill that includes increases to federal cancer and health programs [...]
Nat’l Advocacy Summit Registration Open!
REGISTER TODAY for Lung Cancer Alliance's 10th Anniversary National Advocacy Summit, July 10-12! Don't miss this special opportunity to join fellow lung cancer survivors and advocates in Washington, DC, as we amplify the national call for lung cancer research, early detection and access to high quality of care. At the National Advocacy Summit, you [...]
Take Action for Int’ Women’s Day!
We remember, celebrate and honor the women who have gone before us, touch our lives today and will impact the future tomorrow. Thursday, March 8th is International Women's Day, a time to stand up for the women in our lives. Lung cancer kills more women than all gynecological cancers [...]
A Message from the LCA Board Chair – Lung Cancer: A Women’s Health Imperative
By: Cheryl Healton, DrPH - Director of the Global Institute of Public Health, Dean of College of Global Public Health, New York University Lung cancer is the leading cause of cancer death of women both in the United States and around the world. It claims more lives of women [...]
Lung Love Run/Walk Portland, June 23!
Lung Love Run/Walk Portland is back and better than ever! Join the lung cancer community and Lung Cancer Alliance on Saturday, June 23rd, 2018 at Laurelhurst Park for a day of family-fun as we walk a 5K to fight lung cancer! Mark your calendars, call your family and friends, [...]
#SaveHerLungs
Lung cancer, the leading cancer killer of women, behaves differently in women than men. But we don’t know why. In order to ignite federal government action around this critical issue to advance research breakthroughs, improve quality of life and increase survival for women and the entire lung cancer community, [...]
Finding Hope in Unexpected Places
By Curt Hammock As I approached Christmas in 2010, I reflected on just how good I had it. My business was growing, I was riding my motorcycle, playing golf and getting ready to celebrate my wife, Elaine’s, birthday that month. During that time, I had a constant nose bleed [...]
First Immunotherapy Treatment Approved for Stage III
Friday, February 16, 2018. Today the Food and Drug Administration (FDA) approved Imfinzi (durvalumab), the first immunotherapy drug for stage III non-small cell lung cancer. This approval is what is called "maintenance" therapy, which is when you take a drug to help prevent cancer from growing or coming back [...]
Valentine’s Day With My Hero
By Teri Schrul I feel lucky that I can spend Valentine’s Day with my hero. Not many people can say that. My husband Bill was diagnosed with stage 4 lung cancer in June 2012. It came as a complete shock when we got the news. Five and a half [...]
A Breath Test Leading to Global Access to Care
By Dr. Robert Smyth, MB, MSc As a trainee in pulmonary medicine, lung cancer has long interested me. It is the leading cancer killer worldwide and estimated to be responsible for 1.6 million deaths, about a fifth of all deaths caused by cancer. The highest estimated rates of lung [...]
We Challenge You! Join the Your Cancer Game Plan Challenge!
We’re excited to announce that we’ve joined Merck on the Your Cancer Game Plan Challenge, a social sharing program that will help us raise funds and continue our support for lung cancer patients, caregivers, healthcare providers and others. And we’d like to challenge YOU to join our team. During [...]
LCA Praises Bipartisan, Bicameral Congressional Leadership on Women’s Health Imperative
Senators Marco Rubio (R-FL) and Dianne Feinstein (D-CA) and Representatives Frank LoBiondo (R-NJ), Rick Nolan (D-MN), Barbara Comstock (R-VA) and Suzanne Bonamici (D-OR) Bring Priority Focus to Accelerated Research and Screening Services for Women Impacted by Lung Cancer January 30, 2018 – Today, Lung Cancer Alliance (LCA) hailed the reintroduction [...]
How to Ask for Help
A lung cancer diagnosis can be overwhelmingly emotional and complicated. To provide support during this confusing period, Lung Cancer Alliance started a national HelpLine dedicated exclusively to lung cancer. Caring, trained support professionals answer calls to help patients, caregivers and loved ones understand their lung cancer diagnosis, available treatment [...]
VA-PALS – Improving Lung Cancer Screening For Our Veterans
By: Angela Criswell, Senior Manager of Medical Outreach Lung cancer among veterans is a serious issue because they face higher incidence rates of the disease and lower survival rates compared with civilian populations. The disproportionate impact of lung cancer on veterans is due in part to higher smoking prevalence [...]
FDA expands approval of Gilotrif (afatinib) for NSCLC
Tuesday, January 16, 2018 – The FDA expanded approval of Gilotrif (afatinib), a first-line treatment for patients with non-small cell lung carcinoma (NSCLC) that is epidermal growth factor receptor (EGFR) mutation-positive. Patients with lung cancers with EGFR S768I, L861Q and G719X may now take Gilotrif. Gilotrif, an oral, once-daily [...]
Get Ready for a Groundbreaking Year!
Welcome to 2018! We have so much to look forward to, but there is also a lot of hard work ahead. No matter where you find yourself on your journey -- recently diagnosed, in treatment or a 20-year survivor – Lung Cancer Alliance offers you a home for support [...]
A Message from Our President
Dear Friend, As 2017 prepares to come to a close, let’s take this moment to reflect upon what we have accomplished to improve survivorship, provide support and bring our community together in a lasting and meaningful way. Together we secured more federal research funding, helped patients navigate clinical trials [...]
Starting 2018 With a Full Tank
By Kristin Bramell, Director of Philanthropy, Lung Cancer Alliance 2017 saw its share of successes, but it has not changed the fact that 427 people die each day of lung cancer. The New Year offers hope for members of our community, including those at risk and survivors of this deadly [...]
5 Tips for Helping Your Loved One with Lung Cancer
When a loved one finds out they have lung cancer, it can be hard to know how best to help them, what to say or even how they are feeling. Here are some tips to keep in mind when helping a love one through their lung cancer journey. Take [...]
Science & Research Advances in 2017
By Jennifer C. King, PhD, Director of Science & Research at Lung Cancer Alliance Research on the treatment of lung cancer has been moving quickly and 2017 was no different. Precision medicine was a key theme this year - there have been many treatment advances that depend on the [...]
LCA Participates in Inaugural Meeting of National Lung Cancer Roundtable
Tuesday, December 12, 2017 – Today, Laurie Fenton Ambrose, President and CEO of Lung Cancer Alliance (LCA), along with a coalition of leading healthcare professionals, patients, government and non-governmental organizations, is joining the American Cancer Society for the inaugural meeting of the National Lung Cancer Roundtable. The event, taking [...]
2017 Support Group Facilitator Award
Tuesday, December 12, 2017 -- We are thrilled to announce Kerri Susko, LISW-CP of Greenville Health System, SC as the recipient of LCA's 2017 Support Group Facilitator Award. Each year the award is given to a lung cancer support group facilitator who goes above and beyond to bring support [...]
Preserve Access to Community-Based Care
With just days remaining in the legislative calendar, Congress could leave town without fixing an error in Medicare reimbursements that could make it difficult for people with lung cancer and Medicare insurance coverage to receive community-based treatments. Lung Cancer Alliance is working alongside the Community Care Access Coalition – [...]
Former Collegiate Athlete and Lung Cancer Survivor Finds New Focus as Patient Advocate
Lung cancer doesn’t discriminate. Regardless of age, gender, athleticism or overall health, lung cancer can impact any one of us. Just ask lung cancer survivor Taylor Bell Duck. In 2005, Taylor Bell Duck graduated high school and expected to play Division 1 soccer. But when she got to college, [...]
November is Just the Beginning
LCAM 2017 This November was the most impactful Lung Cancer Awareness Month (LCAM) to date! With your efforts, time and ongoing support we were able to reach more people, raise more awareness and ignite more change than ever before. Thanks to all those who came out to an LCAM [...]
FDA Approves and CMS Advances Coverage Decision for Test that Informs Lung Cancer Treatment Decisions
The Food and Drug Administration has approved the first diagnostic test for cancer-causing mutations in multiple genes at one time in many types of cancer that will help inform treatment options. For patients with lung cancer this means that fewer tests are necessary to determine what drugs are best to [...]
Coping with Fatigue
This week we held the fourth and final Coping Series webinar of 2017, addressing one of the most common symptoms and side effects experienced by lung cancer patients and survivors: fatigue. Over 90% of cancer patients experience fatigue during treatment and around 35% continue to experience fatigue up to [...]
Awareness Happens All Year
Awareness during Lung Cancer Awareness Month is important, but it is vital that the campaign continue all year. Chris Davis, LCA's manager of media relations, provided helpful tips for individual awareness efforts to Mediaplanet. Read on.
Animated Feelings
Nina Beaty was diagnosed with small cell lung cancer (SCLC) in January 2014 after a low-dose CT scan. More than a year later, she enrolled in an immunotherapy clinical trial using the drug Opdivo. As a granddaughter of Broadway composer Richard Rodgers, Nina comes by her creativity naturally. She [...]
Steps in the Right Direction – The Changing Face of Lung Cancer
Jennifer C. King, PhD, Director of Science & Research at Lung Cancer Alliance, sits down with Research!America to discuss the impact research has had on the lung cancer community and what's to come down the line. Read on!
A Lung Cancer Hero at Home
Sheila Ross, a pioneering lung cancer advocate who founded Lung Cancer Alliance’s presence on Capitol Hill, was recently profiled by the Annapolis, Maryland-based Capital Gazette. The article traces both Sheila’s cancer journey and the advocacy measures that brought heightened awareness and landmark legislation on low dose CT screening for [...]
A Veteran Versus Cancer
Ed and his dog, Molly. By Ed Fox, U.S. Army Veteran and Lung Cancer Survivor Lung cancer has been on my radar for some time now because both my father and sister died from this disease. I did everything I possibly could to prevent it: regular screenings, [...]
Marilu Henner Talks Lung Cancer on Today
Tuesday, November 7, 2017 - Today, actress Marilu Henner shared her personal story as a lung cancer caregiver on Megyn Kelly Today, the third hour of NBC's national morning show Today. During Lung Cancer Awareness Month, Henner and her husband, Michael Brown, are opening up about Brown's 2003 diagnosis with [...]
FDA Approves First-Line Treatment for NSCLC with ALK Biomarker
Monday, November 6, 2017 - The United States Food and Drug Administration (FDA) approved Alecensa (alectinib) as a first treatment for advanced non-small cell lung cancer that is positive for a biomarker called ALK. Alecensa was already approved for ALK+ lung cancer that was resistant to other ALK-targeted therapies. [...]
LCA Partners with Memorial Sloan Kettering Cancer Center and the New York University School of Medicine on CASTL Initiative
Monday, November 6, 2017 – Today, Lung Cancer Alliance (LCA) joins the Memorial Sloan Kettering Cancer Center (MSK) and the NYU School of Medicine (NYU) to announce the launch of the Cessation And Screening To Save Lives (CASTL) Initiative. The project, which is supported by funding from the National [...]
In November I Shine a Light!
By Sadie Hansen, Oncology Program Coordinator, Avera Cancer Institute November, next month, is National Lung Cancer Awareness Month. The official color of lung cancer awareness is clear, but it might as well be invisible to most Americans. There is a stigma attached to this diagnosis that is not present [...]
How to Become a Powerful Voice for Justice
Laura Greco was diagnosed in February 2015 with Stage 3A non-small cell adenocarcinoma lung cancer with an ALK mutation after a car accident. Laura had no known risk factors. After her original diagnosis, Laura became a super-advocate for lung cancer research. On November 2, this mother of two young children [...]
In Harvey’s Wake, A Doctor Remembers Houston’s Strength
By Daniel Gomez, MD, Department of Radiation Oncology, Division of Radiation Oncology, MD Anderson Cancer Center Despite the numerous warnings in the week before the storm, I don’t think that many of us grasped how impactful Hurricane Harvey would be. For me, this profound event surprisingly had many parallels [...]
24 Hours of Golf
Curt and his friends at this year's event. By Curt Groebner It all started in mid-summer 2008, when my buddy and I were working our summer job at a local golf course and looking for something fun to do. As a couple of crazy college kids seeking a [...]
2017 National Advocacy Recap
More than 100 lung cancer survivors and advocates united in Washington, DC to ignite change for all those touched by the disease by sharing their personal lung cancer stories with our nation’s leaders at the 9th National Advocacy Summit, which took place on September 27 and 28. Laurie [...]
Story of Hope: Rick Thompson
We were living in Chelmsford, MA at the time of my diagnosis, so we went to Saints Memorial Medical Center in Lowell. It didn't take too long before I was admitted. This was an experience for me, since the last time I was a patient in a hospital was [...]
Story of Hope: Randy Urmstom
I have practiced law in Seattle as of Counsel to Hendricks & Lewis since 1990 and previously worked in legal and engineering positions in the energy, environmental and waste management sectors. I connected with Lung Cancer Alliance (“LCA”) through its predecessor Alliance for Lung Cancer Advocacy Support and Education [...]
Story of Hope: Nancy Alvey
I am a native of Madison WI, having moved to the Louisville KY area in 1981 from Chicago, Illinois. In November of 2005, I was diagnosed with stage II lung cancer in the upper right lobe. Surgery was performed in December 2005 to remove the upper right lobe and [...]
Story of Hope: Kathleen Houlihan
Originally from Texas, I spent twenty years teaching linguistics at the University of Minnesota before moving to New Mexico in 1991 with my husband Jonathan Holt Truex. I had quit smoking shortly before the move. In July of 1998, I developed a pain under my left shoulder blade. My [...]
Story of Hope: Gail Matthews
In October 2000, we were in Aspen, Colorado visiting friends. From there, we flew to the Cooper Clinic in Dallas for an EBT heart scan. I did not want the test as it was uncovered by our health insurance however my husband signed me up anyway. The radiologist read [...]
Story of Hope: Ed Levitt
“I have stage IV lung cancer. It will only win if I let it. I believe attitude and involvement in the cause are everything in staying alive, and staying ahead of this lousy disease.” Prior to being diagnosed with Stage IV lung cancer, my wife and I were making [...]
Story of Hope: Cecilia Izzo
I am Chair of Lung Cancer Alliance's newly formed Washington State Chapter. An eight year (and counting) lung cancer survivor, I am primarily responsible for LCA's activities in Western Washington, including forging relationships with hospitals and medical professionals, fundraising, and public outreach. I am a regular visitor to both [...]
LCA Statement on the American Health Care Act
For many weeks, the country has been awaiting the release of the Republican plan to overhaul the Affordable Care Act (ACA). In anticipation of this effort, Lung Cancer Alliance (LCA) led a coalition effort of lung cancer advocacy groups and sent a letter to Congress in January that outlined [...]
LCA Funds Research to Identify Pathways to Targeted Therapies
Today, Lung Cancer Alliance (LCA) is proud to announce Dr. Robert Smyth, MB, MSc as the recipient of the 2017 Conquer Cancer Foundation (CCF) – Lung Cancer Alliance Young Investigator Award (YIA). Through this award, LCA will support research that examines whether mutations in tumors can be identified through [...]
National Lung Cancer Roundtable Forms to Increase Screening and Reduce Lung Cancer Deaths
Lung Cancer Alliance and a coalition of leading professional, government and non-governmental organizations joined the American Cancer Society to announce they will be coming together to form the National Lung Cancer Roundtable to accelerate the nation's efforts to reduce mortality from lung cancer. The group will focus on ensuring [...]
Where’s My Pink Army?
By Jenny White Jenny at the White House during the National Advocacy Summit. I was in pure shock when my surgeon told me “it’s lung cancer,” while in recovery after video-assisted thoracic surgery (VATS) that was performed to find out what was growing in the right upper [...]
FDA Approves First Combination of Chemotherapy and Immunotherapy for Lung Cancer Patients
Today, the Food and Drug Administration (FDA) approved the first combination of chemotherapy and immunotherapy for patients with lung cancer. Patients with metastatic, nonsquamous, non-small cell lung cancer can now take Keytruda (pembrolizumab) immunotherapy along with chemotherapy (pemetrexed and carboplatin) as a first line of treatment for their lung [...]
Story of Hope: Tom Murphy
Tom Murphy is a lung cancer survivor outside of Baltimore, Maryland. He was diagnosed with stage 1a lung cancer in 2010. This past November, he ran/walked the Philadelphia Half-Marathon with his nurse, Lisa Smith from St. Agnes Hospital. Why did you think you were at risk? First, I had [...]
Honoring Our Champion: Richard Heimler 1960 – 2017
A special message from Laurie Fenton Ambrose, President and CEO of Lung Cancer Alliance It is with a very, very heavy heart that I share the news that our beloved board member, Richard Heimler, passed away on Saturday. We have been in touch with the Heimler family and [...]
15 Ways to Shine a Light on Lung Cancer this November
Fall is here and November is just around the corner which means it is time to get planning for Lung Cancer Awareness Month. This is a time for our community to unite forces to raise awareness, funds and hope to honor those we have lost, support those fighting and help [...]
When Lightning Strikes Twice
Lara (far left) and her children. By Lara Blair, RN 11 years ago, when I was 39 years old, after having spent six months caring for my husband, Bill, who had been diagnosed with malignant thymoma, I suddenly developed a dry hacking cough. In the preceding months, I [...]
Why I’m Riding My Bike from Coast to Coast
John and his Mom By John Matthews Mom had a nagging cough; one that simply wouldn’t go away. I didn’t think there was anything to worry about. A couple of tests, some medicine and she would soon feel better. At the time, she was almost 80 years-old, [...]
Easy Ways to Relax Today!
Relaxation Day is today! Who knew?!? What a great excuse to kick back, calm down and do something nice for yourself. When it comes to facing cancer and the process that comes with it, it can be easy to forget how to relax. Taking care of yourself and your [...]
Workout for the Cause
Mike, his dad and sister during their time in Africa. By Mike Suskin Two things I admired in my father, and I try to exhibit these traits in my own life, were his strong work ethic and an ability to put things into perspective. While he was [...]
Coping Series: Anxiety Before, During and After Lung Cancer Treatment
If you have lung cancer, it is likely you have experienced anxiety. Anxiety is among the most problematic issues of those living with the disease. On Monday, we held the third in our Coping Webinar Series, focusing on anxiety before, during and after lung cancer treatment. We were joined by [...]
Every Day is Parents’ Day
By JoAnn DeCesaris Wellington My father was the type of person you couldn’t miss when he walked in a room. It wasn’t that he was a big “talker” but rather when he did speak it was something you wanted to hear. Geaton A. DeCesaris, Jr. was kind, smart, generous [...]
A Second Chance
By Gary Stumpf Gary hooks success. As with many, I accidentally found my lung cancer. Last fall, a persistent cough brought me to the doctor (thanks to my wife’s insistence). A CT scan, followed by a lung biopsy, confirmed I had stage 4 adenocarcinoma. Further testing found [...]
Beat the Heat!
Summer is heating up and that can play a role in cancer treatment side effects. While going through lung cancer treatment, such as chemotherapy, radiation, targeted therapies or surgery, it is important to find ways to escape the heat. Here are some helpful tips to keep you cool in [...]
2,800 Miles to the Summit
Mary and her husband By Rev. Dr. Mary Holder Naegeli Rev. Dr. Mary Naegeli serves as the Associate Chaplain at John Muir Medical Center in California. She is also an almost-four-year lung cancer survivor. She uses her own experience with cancer to help guide and support patients [...]
Survivor Spotlight: Vincent
Vincent getting ready to take a ride on his longboard. By Vincent Wuellner “There was an element of chance in my lung cancer diagnosis. A coworker noticed a lump in the skin at the base of my neck and advised me to have it looked at. It [...]
My Dad with the Will of Steel
Robert and Kasey on Kasey's wedding day. By Kasey Grizzard Four years ago, my father, Robert Grizzard, was diagnosed with stage IV Adenocarcinoma. This is the type of news that no one wants to hear about a loved one, especially a parent. It hits you like a [...]
We Are Stronger Together
Nicole with her mom, dad and brother shortly after her mom was diagnosed. By Nicole Lindgren My mom was diagnosed with stage 3 lung cancer at the age of 50. She had a persistent cough that just wouldn’t go away. After a number of false diagnoses and [...]
This One’s For Sunny
Sunny by Deena Cook We met Sonia “Sunny” Janin at the first Lung Cancer Alliance National Advocacy Summit we attended in September 2014. This event changed my life and Sunny was an important part of that change. There were five other women from Maryland in our group; [...]
ASCO 2017: What You Need to Know
By Jennifer King, PhD, Director of Science and Research Lung Cancer Alliance staff, Emily Eyres, Andrew Ciupek, Jennifer King and Lanni Boyd, at ASCO. The Lung Cancer Alliance team just returned from the biggest scientific meeting of the year for those who treat patients with cancer, the [...]
“I Found Strength I Never Knew I Had”
Geri celebrates her 10 year anniversary of lung cancer survivorship! By Geri Mangas My story began exactly 10 years ago today, June 4th, 2007, with a right lower lobectomy. I discovered I had stage 2A non-small cell lung cancer during a routine exam by my cardiologist. At the [...]
I Decided I Was Going to Live Life
By Robert Grizzard I was diagnosed on June 7, 2013 at age 62 with stage IV Adenocarcinoma. I had been preparing for my retirement when cancer reared its ugly head. I wasn’t ready to give up on life. On my first visit with my oncologist, he said that the best [...]
My Lucky Break!
Dann (center) at Lung Love Run/Walk Portland. By Dann Wonser Eleven years ago, in 2006, I got the luckiest "break" of my life: I broke my ribs. While that may not seem all that lucky, those broken ribs led to an X-Ray, which led to the discovery [...]
Cancer Has Allowed Me to Live
By Trish Strulson Trish and George I am a survivor, fighter, traveler and explorer. Three and a half years ago I was none of these. My daughter, Taylor, had the difficult task of telling me I had lung cancer. I was only 44 years old. The surgeon [...]
Life Lessons From a Nurse
April Plank, DNP By April Plank, DNP, The Center for Lung Cancer Screening and Prevention, Stony Brook Medicine I realized at a young age that I enjoyed working with people. This rather broad statement could have led me down endless career paths. My decision to choose the [...]
Coping Series: Digestive Problems
Last week we held the second discussion in our Coping Webinar Series, focused on the most common side effects of lung cancer treatment, digestive problems, including nausea, vomiting, constipation and diarrhea. We sat down with Director of Palliative Care at Carolinas Healthcare System, Niki Koesel ANP, ACHPN, FPCN and [...]
Four Easy Ways to Make a Difference This May!
It doesn’t take much to make a big difference in the life of someone impacted by lung cancer. Here are a couple easy ways to put a voice and a face to lung cancer in your local community and beyond! Send a Letter to Your Local Newspaper – Your [...]
What We’ve Done These First 100 Days and What You Can Do!
By Elridge Proctor, MPA, Director of Health Policy Spring is here and, yet, we are only just beginning the most serious part of Congress’ work which includes establishing budget priorities for this year. Furthermore, Congress will need to decide on funding for the rest of Fiscal Year (FY) 2017, [...]
A Message from LCA’s Board Chair
Cheryl Healton By Cheryl G. Healton, Dr. PH Nice to meet you! Although I just took the role of Board Chair, the truth is, I’ve been here alongside you for over a decade since I joined Lung Cancer Alliance’s Board of Directors in 2005. I’ve spent my [...]
Volunteer. The Reward is Twofold.
Michelle and her daughter pose with the Philly Phanatic at Lung Love Run/Walk Philly. By Michelle Russo Up until the fall of 2010, I had never heard of Lung Cancer Alliance (LCA). I was still in the throes of my husband’s sudden onset of lung cancer and [...]
Yes, you CAN make a difference in research
Jim and his family. By Jim Pantelas My wife was six-and-a-half months pregnant on the day I was diagnosed with stage 3B, non-small-cell lung cancer. That was eleven years ago and I’ve been dancing with NED (no evidence of disease) for the past ten years. But dancing [...]
My Purpose, Our Purpose
Ken and Sheila By Ken Wheatley As a caregiver and now widower, lung cancer radically changed the course of my life and my daily existence. I had finally found the most perfect woman. “Soulmate” tends to be an overused word, but in this case it was completely [...]
With Every Breath We Take
Brad and Lana By Brad Thacker I was blessed with a beautiful, fun and kind sister, Lana. Although she was eight years older, we were very close. We lost my mother to breast cancer when I was in college and Lana took me under her wing and [...]
Survivor Spotlight: Maureen
Maureen with her daughter on her 21st birthday. In the early spring of 2011 I was on my first 6-mile outdoor run of the season. My hip was really bothering me. Two weeks later my husband insisted that I go see the orthopedist for my, “sports injury.” After several [...]
Coping Series: Shortness of Breath
This month we kick off our Coping Webinar Series, a program to help you manage the side effects and symptoms of lung cancer and its treatment. Our first webinar focused on shortness of breath, an incredibly common issue for those living with lung cancer. We sat down with nurse [...]
Survivor Spotlight: Ide
By Ide Mills The day after Thanksgiving in 2010 I saw my internist for discomfort in my throat that I couldn’t clear. She thought allergies, while I thought everything else, BUT cancer. Over the next few months, it became increasingly more difficult to complete a sentence without being short [...]
A Tribute to Sheila Ross – Our Very Own Unsinkable Molly Brown
Sheila Ross on Capitol Hill By Laurie Fenton Ambrose, President & CEO of Lung Cancer Alliance It is with enormous pride and with my deepest admiration and respect that I talk to you about Lung Cancer Alliance’s very own Unsinkable Molly Brown, Sheila Ross, who after almost [...]
Living Life AC (After Cancer)
By Deborah Benton, 15 year lung cancer survivor. 15 year lung cancer survivor, Deborah Benton It’s a new year! For me, it is the year of my 15th birthday. It is really the year of my 59th birthday, but thanks to lung cancer, my life has been [...]
One Magic Pill and 24 Months Later
By Michael McCarty, lung cancer survivor and advocate Michael in a meeting on the Hill, September 2014 It was a hot day in early August, 2012 and I was out running and ran into some friends on the road and decided to join them. I knew for [...]